Please refer to the Classification Of Elements And Periodicity In Properties Revision Notes given below. These revision notes have been designed as per the latest NCERT, CBSE and KVS books issued for the current academic year. Students will be able to understand the entire chapter in your class 11th Chemistry book. We have provided chapter wise Notes for Class 11 Chemistry as per the latest examination pattern.
Revision Notes Chapter 3 Classification Of Elements And Periodicity In Properties
Students of Class 11 Chemistry will be able to revise the entire chapter and also learn all important concepts based on the topic wise notes given below. Our best teachers for Grade 11 have prepared these to help you get better marks in upcoming examinations. These revision notes cover all important topics given in this chapter.
Mandeleev’s Periodic Law:- The properties of the elements are the periodic function of their atomic masses.
Moseley, the English physicist showed that atomic number is more fundamental property of an element than its atomic mass. Therefore, the position of an element in the periodic table depends on its atomic number than its atomic mass.
Modern Periodic Law: The physical and chemical properties of elements are the periodic functions of their atomic numbers.
Types of Elements: s-, p-, d- and f- blocks.
MAIN GROUP ELEMENTS/ REPRESENTATIVE ELEMENTS:
The s- and p- block elements are called main group elements or representative elements.
s- block elements: Group-1 (Alkali metals) and Group-2 elements (Alkaline earth metals) which respectively have ns1 and ns2 outermost electronic configurations.
p- Block elements: They belongs to group- 13 to 18. The outer most electronic configuration is ns2 np1-6. He (1s2) is a s- block element but is positioned with the group 18 elements ( ns2 np6) because it has completely filled valence shell and as a result, exhibits properties characteristic of other noble gases.
d- block elements (Transition elements) are the elements of group 3 to 12 having outer electronic configuration (n-1) d1-10 ns1-2. Four transition series are 3d, 4d, 5d and 6d. The 6d- series is incomplete. Atomic radius generally decreases across a period and increases as we descend the group.
f-Block elements (Inner- transition Series)
Lanthanoids charecterised by the filling of4 f-orbitals, are the elements following lanthanum from 58Ce to 71Lu. Actinoids characterised by filling of 5f-orbitals, are the elements following actinium from 70 Th to 103Lr. Characteristic outer electronic configuration is (n-2) f1-14 (n-1) d0-1 ns2.
Noble Gases: The gaseous elements of group 18 are called noble gases. The general outer most electronic configuration of noble gases (except He) is ns2 np6. He exceptionally has 1s2 configuration. Thus the outermost shell of noble gases is completely filled.
PERIODICITY: The repetition of similar properties after regular intervals is called periodicity.
Cause of Periodicity: The properties of elements are the periodic repetition of similar electronic configuration of elements as the atomic number increases.
ATOMIC PROPERTIES: The physical characteristics of the atom of an element are called atomic properties. The properties such as atomic radius, ionic radius, ionisation energy, electro-negativity, electron affinity and valence etc. called atomic properties.
ATOMIC RADIUS: The distance from the centre of the nucleus to the outermost shell of the electrons in the atom of any element is called its atomic radius.
Periodicity:
(i) In period- Atomic radius of elements decreases from left to right in a period.
(ii) In Group- Atomic radius of elements increases on moving top to bottom in a group.
COVALENT RADIUS: Half the inter-nuclear distance between two similar atoms of any element which are covalently bonded to each other by a single covalent bond is called covalent radius.
VAN DER WAALS’ RADIUS: Half the inter-nuclear separation between two similar adjacent atoms belonging to the two neighbouring molecules of the same substance in the solid state is called the van der waals’radius of that atom.
METALLIC RADIUS: Half the distance between the nuclei of the two adjacent metal atoms in a close packed lattice of the metal is called its metallic radius. Van der Waals’radius > Metallic radius > Covalent radius
IONIC RADIUS: The effective distance from the centre of the nucleus of an ion upto which it has an influence on its electron cloud is called its ionic radius.A cation is smaller but the anion is larger than the parent atom. In case of isoelectronic species, the cation with greater positive charge has smaller radius but anion with greater negative charge has the larger radii.
IONISATION ENTHALPY: The ionisation enthalpy is the molar enthalpy change accompanying the removal of an electron from a gaseous phase atom or ion in its ground state. Thus enthalpy change for the reaction;
M(g) → M+ (g) + e–
Is the ionisation enthalpy of the element M. Like ionisation energies for successive ionisation, the successive ionisation enthalpy may also be termed as 2nd ionisation enthalpy (ΔrH2), third ionisation enthalpy (ΔrH3) etc. The term ionisation enthalpy is taken for the first ionisation enthalpy, (ΔrH1) is expressed in kg mol– or in eV.
Periodicity:
(i) Generally the ionisation enthalpies follow the order ( there are few exceptions):
(ΔrH1) < (ΔrH2) < (ΔrH3)
(ii) The ionisation enthalpy decreases on moving top to bottom in a group.
(iii)The ionisation enthalpy increases on moving from left to right in a period.
ELECTRON GAIN ENTHALPY: The electron gain enthalpy (ΔegH) is the molar enthalpy change when an isolated gaseous atom or ion in its ground state adds an electron to form the corresponding anion thus the enthalpy change for the reaction;
X(g) + e– → X–(g)
Is called the electron gain enthalpy (ΔegH) of the element X. The Δeg H may be positive or negative.
The successive values for the addition of second, third etc. Electron, these are called second, third etc. electron gain enthalpies. For example,
X(g) + e– → X–(g) ΔH= Δeg H1 is called first electron gain
enthalpy
X–(g) + e– → X2-(g) ΔH= ΔegH2 is called second electron gain
enthalpy
X2-(g) + e– → X3-(g) ΔH= ΔegH3 is called third electron gain
enthalpy Usually the term electron gain enthalpy (ΔegH) means the first electron gain enthalpy.
Periodicity:
(i) In period- The electron gain enthalpy increases from left to right in a period.
(ii) In group- The electron gain enthalpy decreases from top to bottom in a group.
ELECTRONEGATIVITY: “The relative tendency of an atom in a molecule to attract the shared pair of electrons towards itself is termed as its electronegativity.”
Periodicity:
(i) In period- The electro-negativity increases from left to right in a period.
(ii) In group- The electro-negativity decreases from top to bottom in a group.
VALENCE ELECTRONS: The electrons present in outermost shell are called as valence electron. Because the electrons in the outermost shell determine the valency of an element.
VALENCY OF AN ELEMENT: The number of hydrogen or halogen atom or double the number of oxygen atom, which combin with one atom of the element is taken as its valency. According to the electronic concept of valency, “ the number of electrons which an atom loses or gains or shares with other atom to attain the noble gas configuration is termed as its valency.”
Periodicity:
(i) In period- The valency first increases then decreases from left to right in a period.
(ii) In group- The valency remains constant from top to bottom in a group.
ELECTROPOSITIVE OR METALLIC CHARACTER: The tendency of an element to lose electrons and forms positive ions (cations) is called electropositive or metallic character. The elements having lower ionisation energies have higher tendency to lose electrons, thus they are electropositive or metallic in their behaviour. Alkali metals are the most highly electropositive elements.
Periodicity:
(i) In period- The electropositive or metallic characters decreases from left to right in a period.
(ii) In group- The electropositive or metallic characters increases from top to bottom in a group.
ELECTRO-NEGATIVE OR NON- METALLIC CHARACTERS: the tendency of an element to accept electrons to form an anion is called its non metallic or electronegative character. The elements having high electro-negativity have higher tendency to gain electrons and forms anion. So, the elements in the upper right hand portion of the periodic table are electro-negative or non-metallic in nature.
Periodicity:
(i) In period- The electro-negative or non- metallic characters increases from left to right in a period.
(ii) In group- The electro-negative or non-metallic characters decreases from top to bottom in a group.
REACTIVITY OF METALS:
(i) In period- The tendency of an element to lose electrons decreases in a period. So the reactivity of metals decreases from left to right in a period.
(ii) In group- The tendency of an element to lose electrons increases in a period. So the reactivity of metals increases from top to bottom in a group.
REACTIVITY OF NON- METALS:
(i) In period- The tendency of an element to gain electrons increases in a period. So the reactivity of non-metals increases from left to right in a period.
(ii) In group- The tendency of an element to gain electrons decreases in a group. So the reactivity of non-metals increases from top to bottom in a group.
SOLUBILITY OF ALKALI METALS CARBONATES AND BICARBONATES:
PERIODICITY IN GROUP: The solubility of alkali metal carbonates and bicarbonates in water increases down the group (From Lithium to Caesium).
SOLUBILITY OF ALKALINE EARTH METAL HYDROXIDES AND SULPHATES:
PERIODICITY IN GROUP: The solubility of alkaline earth metal hydroxide and sulphates in water increases down the group (From Beryllium to Barium).
BASIC STRENGTH OF ALKALINE EARTH METAL HYDROXIDES:
PERIODICITY IN GROUP: The basic strength of alkaline earth metal hydroxide in water increases down the group (From Beryllium to Barium), i.e.,

THERMAL STABILITY OF CARBONATES OF ALKALI AND ALKALINE EARTH METALS:
Except lithium carbonate, (LiCO3), the carbonates of all other alkali metals are stable towards heat, i.e., carbonates of alkali metals (except LiCO3) do not decompose on heating. LiCO3 decomposes on heating to give lithium oxide (LiCO3) The carbonates of alkaline earth metals are relatively less stable. On heating, they decompose to give corresponding oxide and CO2 gas. The decomposition temperature for alkaline earth metal carbonates increases as we go down the group.
Anomalous Properties of Second Period Elements
Their anomalous behaviour is attributed to their small size, large charge/radius ratio, high electro negativity, non- availability of d- orbitals in their valence shell. the first member of each group of p-Block elements displays greater ability to form pp-pp multiple bonds to itself (e.g. C=C, C≡C O=O, N≡N) and to other second period elements (e.g. C=O, C≡N, N=O) compared to subsequent member of the group.
Important Points
1. Dobereiner’s Triads: In 1817 a German chemist Doberneiner identified certain groups of three elements. These groups of three elements having similar properties was called triads. When three elements were arranged in order of their increasing atomic masses, the atomic mass of the middle element was roughly the mean of the atomic masses of the other two element
2. New Lands Law of octaves: When elements were arranged in order of their increasing relative atomic masses. The properties of every eight elements were similar to the first one, like the eighth note of a musical scale.This repetition in the properties of elements is just like the repetition of eighth node in an octave of music.
3. Mendeleev’s Periodic Law:The physical and chemical properties of elements are the periodic function of their atomic masses.
4. Mendeleev’s Periodic Table:When mendeleev started his work, 63 elements were known at that time. He selected hydrogen and oxygen as they are very reactive and formed compounds with most elements. Mendeleev’s periodic table contains vertical columns called groups and horizontal rows called periods. There were 7 periods and 8 groups. Noble gases were not known at that time. So there was no group of noble gases.The elements in each group of the periodic tables are similar to one another in many properties. The similar properties of the elements are repeated periodically
Merits of mendeleev’s classification
• Mendeleev’s periodic law predicted the existence of some elements that had not been discovered at that time
• .Could predict the properties of several elements on the basis of their position in the periodic table.
• Could accommodate noble gases when they were discovered.
Limitations of mendeleev’s classification :-
• The correct position could not be assigned to the hydrogen in the periodic table.
• Wrong order of the atomic masses of some elements could not be explained.
• The position of isotopes could not be explained.
• Uncertainty in prediction of new elements was there.
5. Modern periodic law: Properties of elements are the periodic function of their atomic number.
6. Modern Periodic Table: This table was prepared was Bohr and is based upon the electronic configuration of elements . The table consists of 18 vertical columns called groups Elements having similar outer electronic configurations in their atoms are arranged in vertical columns, referred to as groups . According to the recommendation of International Union of Pure and Applied Chemistry (IUPAC), the groups are numbered from 1 to 18 and the table consists of 7 horizontal rows called periods. The first period contains 2 elements. The subsequent periods consists of 8, 8, 18, 18 and 32 elements, respectively. The seventh period is incomplete and like the sixth period would have a theoretical maximum (on the basis of quantum numbers) of 32 elements. In this form of the Periodic Table, 14 elements of both sixth and seventh periods (lanthanoids and actinoids, respectively) are placed in separate panels at the bottom
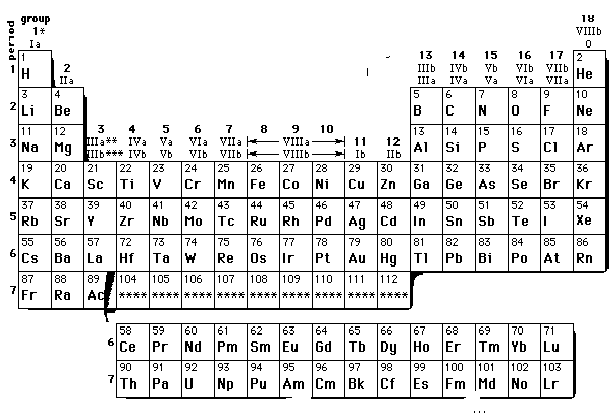
7. .Notation for IUPAC Nomenclature of Elements With Z > 100
8. We can classify the elements into four blocks viz., s-block, p-block, d-block and f-block depending on the type of atomic orbital that are being filled with electrons.
9. s-Block Elements :The elements of Group 1 (alkali metals) and Group 2 (alkaline earth metals) which have ns1and ns2 outermost electronic configuration belong to the s-Block Elements.
10. p-Block Elements :The p-Block Elements comprise those belonging to Group 13 to 18 and these together with the s-Block Elements are called the Representative Elements or Main Group Elements. The outermost electronic configuration varies from ns2np1 to ns2np6 in each period.
11. d-Block Elements: These are the elements of Group 3 to 12 in the centre of the Periodic Table. These are characterised by the filling of inner d orbitals by electrons and are therefore referred to as d-Block Elements. These elements have the general outer electronic configuration (n-1)d1-10ns0-2.
12. f-Block Elements The two rows of elements at the bottom of the Periodic Table, called the Lanthanoids, Ce(Z = 58) – Lu(Z = 71) and Actinoids, Th(Z = 90) – Lr (Z = 103) are characterised by the outer electronic configuration (n-2)f1-14 (n-1)d0-1ns2. The last electron added to each element is filled in f- orbital. These two series of elements are hence called the Inner- Transition Elements (f-Block Elements).
13. Variation in Atomic Radius in Period: The atomic size generally decreases across a period It is because within the period the outer electrons are in the same valence shell and the effective nuclear charge increases as the atomic number increases resulting in the increased attraction of electrons to the nucleus.
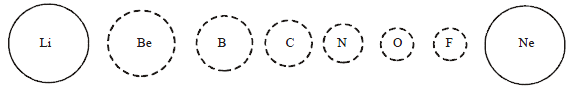
14. Variation in Atomic Radius in Group: Within a family or vertical column of the periodic table, the atomic radius increases regularly with atomic number as). as we descend the groups, the principal quantum number (n) increases and the valence electrons are farther from the nucleus. This happens because the inner energy levels are filled with electrons, which serve to shield the outer electrons from the pull of the nucleus. Consequently the size of the atom increases as reflected in the atomic radii.
15. The atomic radii of noble gases are not considered here. Being monatomic, their (non-bonded radii) values are very large. In fact radii of noble gases should be compared not with the covalent radii but with the van der Waals radii of other elements.
16.A cation is smaller than its parent atom because it has fewer electrons while its nuclear charge remains the same. The size of an anion will be larger than that of the parent atom because the addition of one or more electrons would result in increased repulsion among the electrons and a decrease in effective nuclear charge. For example, the ionic radius of fluoride ion (F– ) is 136 pm whereas the atomic radius of fluorine is only 64 pm. On the other hand, the atomic radius of sodium is 186 pm compared to the ionic radius of 95 pm for Na+.
17. Isoelectronic species :Atoms and ions which contain the same number of electrons.. For example, O2–, F–, Na+ and Mg2+ have the same number ofelectrons (10). Their radii would be different because of their different nuclear charges. The cation with the greater positive charge will have a smaller radius because of the greater attraction of the electrons to the nucleus. Anion with the greater negative charge will have the larger radius. In this case, the net repulsion of the electrons will outweigh the nuclear charge and the ion will expand in size.
18. Ionization Enthalpy: It represents the energy required to remove an electron from an isolated gaseous atom (X) in its ground state. In other words, the first ionization enthalpy for an element X is the enthalpy change (ΔiH) for the reaction depicted in equation. X (g)→ X+(g) + e– . The ionization enthalpy is expressed in units of kJ mol–1. We can define the second ionization enthalpy as the energy required to remove the second most loosely bound electron; it is the energy required to carry out the reaction shown in equation X+(g) → X2+(g) + e– .
Energy is always required to remove electrons from an atom and hence ionization enthalpies are always positive. The second ionization enthalpy will be higher than the first ionization enthalpy because it is more difficult to remove an electron from a positively charged ion than from a neutral atom.In the same way the third ionization enthalpy will be higher than the second and so on. The term ―ionization enthalpy‖, if not qualified, is taken as the first ionization enthalpy.
19. Variation in Ionization Enthalpy in Group: As we descend in a group the first ionization enthalpy generally decreases .Because as we go down a group, the outermost electron being increasingly farther from the nucleus, there is an increased shielding of the nuclear charge by the electrons in the inner levels. In this case, increase in shielding outweighs the increasing nuclear charge and the removal of the outermost electron requires less energy down a group
20. Variation in Ionization Enthalpy in Period: The first ionization enthalpy generally increases as we go across a period. When we move from left to right in period, successive electrons are added to orbitals in the same principal quantum level and the shielding of the nuclear charge by the inner core of electrons does not increase very much to compensate for the increased attraction of the electron to the nucleus. Thus, across a period, increasing nuclear charge outweighs the shielding. Consequently, the outermost electrons are held more and more tightly and the ionization enthalpy increases across a period

21. Electron Gain Enthalpy: When an electron is added to a neutral gaseous atom (X) to convert it into a negative ion, the enthalpy change accompanying the process is defined as the Electron Gain Enthalpy (ΔegH). Electron gain enthalpy provides a measure of the ease with which an atom adds an electron to form anion as represented by equation.
X(g) + e– → X–(g) . Depending on the element, the process of adding an electron to the atom can be either endothermic or exothermic. For many elements energy is released when an electron is added to the atom and the electron gain enthalpy is negative. For example, group 17 elements (the halogens) have very high negative electron gain enthalpies because they can attain stable noble gas electronic configurations by picking up an electron. On the other hand, noble gases have large positive electron gain enthalpies because the electron has to enter the next higher principal quantum level leading to a very unstable electronic configuration.
22. Variation in electron gain enthalpies in Group & period: The variation in electron gain enthalpies of elements is less systematic than for ionization enthalpies. As a general rule, electron gain enthalpy becomes more negative with increase in the atomic number across a period. The effective nuclear charge increases from left to right across a period and consequently it will be easier to add an electron to a smaller atom since the added electron on an average would be closer to the positively charged nucleus. We should also expect electron gain enthalpy to become less negative as we go down a group because the size of the atom increases and the added electron would be farther from the nucleus. This is generally the case. However, electron gain enthalpy of O or F is less negative than that of the succeeding element . This is because when an electron is added to O or F, the added electron goes to the smaller n = 2 quantum level and suffers significant repulsion from the other electrons present in this level. For the n = 3 quantum level (S or Cl), the added electron occupies a larger region of space and the electronelectron repulsion is much less.
23. Electronegativity: A qualitative measure of the ability of an atom in a chemical compound to attract shared electrons to itself is called electro negativity Linus Pauling, an American scientist, in 1922 assigned arbitrarily a value of 4.0 to fluorine, the element considered to have the greatest ability to attract electrons. Electronegativity generally increases across a period from left to right (say from lithium to fluorine) and decrease down a group (say from fluorine to astatine) in the periodic table.
24. Anomalous Properties of Second Period Elements: The first element of each of the groups 1 (lithium) and 2 (beryllium) and groups 13-17 (boron to fluorine) differs in many respects from the other members of their respective group. For example, lithium unlike other alkali metals, and beryllium unlike other alkaline earth metals, form compounds with pronounced covalent character; the other members of these groups predominantly form ionic compounds. In fact the behaviour of lithium and beryllium is more similar with the second element of the Group 1, 2 ,13, 14, 15, 16 ,17. following group i.e., magnesium and aluminum, respectively. This sort of similarity is commonly referred to as diagonal relationship in the periodic properties. The anomalous behaviour is attributed to their small size, large charge/ radius ratio and high electronegativity of the elements. In addition, the first member of group has only four valence orbitals (2s and 2p) available for bonding, whereas the second member of the groups have nine valence orbitals (3s, 3p, 3d). As a consequence of this, the maximum covalency of the first member of each group is 4 (e.g., boron can only form[BF4]- , whereas the other members of the groups can expand their valence shell to accommodate more than four pairs of electrons e.g., aluminum forms [AlF6]3- ). Furthermore, the first member of p-block elements displays greater ability to form pΠ – pΠ multiple bonds to itself (e.g., C = C, C ≡ C, N = N, N≡ N) and to other second period elements (e.g., C = O, C = N, C ≡ N, N = O) compared to subsequent members of the same group.



