Please refer to the Chemical Bonding and Molecular Structure Revision Notes given below. These revision notes have been designed as per the latest NCERT, CBSE and KVS books issued for the current academic year. Students will be able to understand the entire chapter in your class 11th Chemistry book. We have provided chapter wise Notes for Class 11 Chemistry as per the latest examination pattern.
Revision Notes Chapter 4 Chemical Bonding and Molecular Structure
Students of Class 11 Chemistry will be able to revise the entire chapter and also learn all important concepts based on the topic wise notes given below. Our best teachers for Grade 11 have prepared these to help you get better marks in upcoming examinations. These revision notes cover all important topics given in this chapter.
OCTET RULE: During a chemical reaction the atoms tend to adjust their electronic arrangement in such a way that they achieve 8 e– in their outermost electron. This is called octet rule.
CHEMICAL BOND: the chemical force which keeps the atoms in any molecule together is called a chemical bond.
IONIC BOND: The columbic force of attraction which holds the appositively charged ions together is called an ionic bond. An ionic bond is formed by the complete transfer of one or more electrons from the atom of a metal to an atom of non: metal.
LATTICE ENTHALPY: The molar enthalpy change accompanying the complete separation of the constituent particles that compose of the solids (such as ions for ionic solid, molecules for molecular solids) under standard conditions is called lattice enthalpy (ΔlHo). The lattice enthalpy is a positive quantity.
ELECTRO VALENCY: The number of electrons lost or gain by an atom of an element is called as electrovalency. The element which give up electrons to form positive ions are said to have positive valency, while the elements which accept electrons to form negative ions are said to have negative valency.
FORMATION OF AN IONIC BOND: It is favoured by,
(i) the low ionisation enthalpy of a metallic element which forms the cations,
(ii) High electron gain enthalpy of non: metallic element which forms the anions,
(iii) Large lattice enthalpy i.e; the smaller size and the higher charge of the atoms.
COVALENCY:The number of electrons which an atom contributes towards mutual sharing during the formation of a chemical bond called its covalency in that compound.
SINGLE COVALENT BOND: A covalent bond formed by the mutual sharing of one pair of electrons is called a single covalent bond, or simply a single bond. A single covalent bond is represented by a small line (−) between the two atoms.
DOUBLE COVALENT BOND: A covalent bond formed by the mutual sharing of two pair of electrons is called a double covalent bond, or simply a double bond. A double covalent bond is represented by two small horizontal lines (=) between the two atoms. E.g. O=O, O=C=O etc.
TRIPLE COVALENT BOND: A covalent bond formed by the mutual sharing of three pair of electrons is called a triple covalent bond, or simply a triple bond. A triple covalent bond is represented by three small horizontal lines (≡) between the two atoms. E.g. N≡N, H-C≡C-H etc.
FORMATION OF A COVALENT BOND: Formation of a covalent bond is favoured by
(i) High ionisation enthalpy of the combining elements.
(ii)Nearly equal electron gain enthalpy and equal electro-negativities of combining elements.
(iii) High nuclear charge and small atomic size of the combining elements.
POLAR COVALENT BOND: The bond between two unlike atoms which differ in their affinities for electrons is said to be polar covalent bond. E.g. H-Cl COORDINATE BOND: The bond formed when one sided sharing of electrons take place is called a coordinate bond. Such a bond is also known as dative bond. It is represented by an arrow (→) pointing towards the acceptor atom. E.g. H3N→BF3
Bond Length: Bond length is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule
Bond Angle: It is defined as the angle between the orbitals containing bonding electron pairs around the central atom in a molecule/complex ion Bond Enthalpy: It is defined as the amount of energy required to break one mole of bonds of a particular type between two atoms in a gaseous state.
Bond Order: In the Lewis description of covalent bond, the Bond Order is given by the number of bonds between the two atoms in a molecule
Resonance: whenever a single Lewis structure cannot describe a molecule accurately, a number of structures with similar energy, positions of nuclei, bonding and non-bonding pairs of electrons are taken as the canonical structures of the hybrid which describes the molecule accurately
Dipole moment : The product of the magnitude of the charge and the distance between the centres of positive and negative charge.It is a vector quantity and is represented by an arrow with its tail at the positive centre and head pointing towards a negative centre. Dipole moment (μ) = charge (Q) × distance of separation (r)
SIGMA BOND: A covalent bond formed due to the overlapping of orbitals of the two atoms along the line joining the two nuclei (orbital axis) is called sigma (σ) bond. For example, the bond formed due to s-s and s-p, p-p overlapping along the orbital axis are sigma bonds.
Pi- BOND: A covalent bond formed by the side wise overlapping of p- or dorbitals of two atoms is called as pi (π) bond. For example, the bond formed due to the sideways overlapping of the two p- orbitals is a pi- bond.
HYDROGEN BOND: The bond between the hydrogen atom of one molecule and a more electro- negative element of same or another molecule is called as hydrogen bond.
HYBRIDIZATION: The process of mixing of the atomic orbitals to form new hybrid orbitals is called hybridization. All hybrid orbitals of a particular kind have equal energy, identical shapes and are symmetrically oriented in shape. The hybrid orbitals are designed according to the type and the atomic orbitals merging together, e.g.,


Important Points
1. Lewis dot structures are shorthand to represent the valence electrons of an atom. The structures are written as the element symbol surrounded by dots that represent the valence electrons.
2. Covalent Bonds: The bond formed between two atoms by mutual sharing of electrons between them so as to complete their octets or duplets. When two atoms share one electron pair they are said to be joined by a single covalent bond.e.g H2 If two atoms share two electron pairs of electrons, the covalent bond between them is called a double bond. e.g O2 If two atoms share three electron pairs of electrons, the covalent bond between them is called a double bond. e.g N2
3. Octet Rule: Kossel and Lewis in 1916 developed an important theory of chemical combination
between atoms known as electronic theory of chemical bonding. According to this, atoms can combine either by transfer of valence electrons from one atom to another or by sharing of valence electrons in order to attain their octet. This is known as octet rule.
4. Limitations of octet rule :
a) Incomplete octet of the central atom: In some compounds the number of electrons surrounding the central atom is less than eight. This is especially the case with elements having less than four valence electrons. Examples- LiCl ,BeCl2 , BCl3
b) Odd-electron molecules: In molecules with an odd number of electrons like nitric oxide, NO and nitrogen dioxide, the octet rule is not satisfied for all the atoms.
c) The expanded octet : Elements in and beyond the third period of the periodic table have, apart from 3s and 3p orbitals, 3d orbitals also available for bonding. In a number of compounds of these elements there are more than eight valence electrons around the central atom. This is termed as the expanded octet. Some of examples of such compounds are: PF5, SF6.
d) This theory does not account for the shape of molecules.
5. Electrovalent bond or Ionic Bond: The chemical bond as result of transfer of electron from one atom (electropositive) to another atom (electronegative).Ionic bonds will be formed more easily between elements with comparatively low ionization enthalpies and elements with comparatively high negative value of electron gain enthalpy. Most ionic compounds have cations derived from metallic elements and anions from non-metallic elements.
6. Formation of Ionic Bond
M(g) → M+(g) + e– ; ionization enthalpy
X(g) + e– → X–(g) ; electron gain enthalpy
M+(g) + X–(g) → MX(s)
7. Bond length: It is defined as the equilibrium distance between the nuclei of two bonded atoms in a molecule.
8. Bond Angle: It is defined as the angle between the orbital containing bonding electron pairs around the central atom in a molecule/complex ion. It gives some idea regarding the distribution of orbital around the central atom in a molecule/complex ion and hence it helps us in determining its shape
9. Bond enthalpy: It is defined as the amount of energy required to break one mole of bonds of a particular type between two atoms in a gaseous state. The unit of bond enthalpy is kJ mol–1
10. Bond Order : The Bond Order is given by the number of bonds between the two atoms in a molecule. E.g.: Bond Order of O2= 2. With increase in bond order, bond enthalpy increases and bond length decreases.
11. Resonance: According to the concept of resonance, whenever a single Lewis structure cannot describe a molecule accurately, a number of structures with similar energy, positions of nuclei, bonding and the non- bonding pairs of electrons are taken as the canonical structures of the hybrid which describes the molecule accurately
12. Polarity of bonds: In case of heteronuclear molecules like HCl, the shared pair of electron between the two atoms gets displaced more towards chlorine since the electronegativity of chlorine is far greater than that of hydrogen. The resultant covalent bond is called a polar covalent bond.
13. Dipole moment: As a result of polarization, the molecule possesses the dipole moment which can be defined as the product of charge and the distance between the centers of positive and negative charge. It is usually designated by a Greek letter ‘μ‘. Mathematically, it is expressed as follows:
Dipole moment (μ ) = charge (Q) X distance of separation (r)
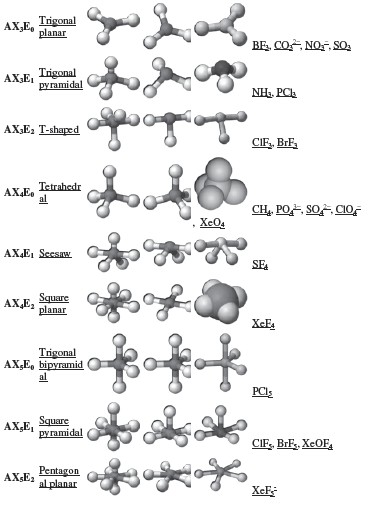
14. VSEPR Theory
• The shape of a molecule depends upon the number of valence shell electron pairs (bonded or nonbonded) around the central atom.
• Pairs of electrons in the valence shell repel one another since their electron clouds are negatively charged.
• These pairs of electrons tend to occupy such positions in space that minimize repulsion and thus maximise distance between them.
• The valence shell is taken as a sphere with the electron pairs localising on the spherical surface at maximum distance from one another.
• A multiple bond is treated as if it is a single electron pair and the two or three electron pairs of a multiple bond are treated as a single super pair.
• Where two or more resonance structures can represent a molecule, the VSEPR model is applicable to any such structure.
• The repulsive interaction of electron pairs decrease in the order: Lone pair (lp) – Lone pair (lp) > Lone pair (lp) – Bond pair (bp) > Bond pair (bp) –Bond pair (bp)
• Geometry of Molecules on the basis of VSEPR Theory

15. Hybridization: It can be defined as the process of intermixing of the orbitals of slightly different energies so as to redistribute their energies, resulting in the formations of new set of orbitals of equivalent energies and shape.
• Salient Features of hybridization :
• The number of hybrid orbitals is equal to the number of the atomic orbitals that get hybridised.
• The hybridised orbitals are always equivalent in energy and shape.
• The hybrid orbitals are more effective in forming stable bonds than the pure atomic orbitals.
• These hybrid orbitals are directed in space in some preferred direction to have minimum repulsion between electron pairs and thus a stable arrangement.
16. Types of Hybridisation
• sp hybridisation- This type of hybridisation involves the mixing of one s and one p orbital resulting in the formation of two equivalent sp hybrid orbitals.e.g.BeCl2
• sp2 hybridisation- In this hybridisation there is involvement of one s and two p-orbitals in order to form there equivalent sp2 hybridised orbitals. e.g.BCl3
• sp3 hybridisation- When there is mixing of one s and three p-orbitals of the valence shell to form four sp3 hybrid orbitals of equivalent energies and shape. e.g.CH4
17. Molecular orbital: It gives electron probability distribution around a group of nuclei in a molecule. They are filled in the same way as atomic orbitals. Molecular orbitals are formed by linear combination of atomic orbitals.
18. Bonding molecular orbital: A molecular orbital that is formed by addition overlap (i.e., when the lobes of atomic orbitals overlap with the same sign) of two atomic orbitals is known as bonding molecular orbital. It is represented as ΨMO = ΨA + ΨB Its energy is lower than the atomic orbitals from which it is formed. It favours bonding.
19. Anti-bonding molecular orbital: A molecular orbital that is obtained by the subtraction overlap (i.e., when the lobes of atomic orbitals overlap with the opposite sign) of two atomic orbitals is know as anti-bonding molecular orbital. It is represented as Ψ*MO = ΨA + ΨB Its energy is higher than the atomic orbitals from which it is formed. It does not favour bonding.
20. Bond order: It is defined as half of the difference between number of electrons in bonding and anti-bonding orbitals, i.e., B.O. = ½ (Nb – Na) ‘where Nb are number of electrons in bonding orbitals‘ and Na are number of electrons in anti-bonding orbitals. Bond order helps in estimating stability of atom.
21. Relationship between electronic configuration and molecular behaviour :
(a) If Nb is greater than Na, the molecule is stable.
(b) The molecule is unstable if Nais greater than Nb .
(c) The molecule is also unstable if Nais equal to Nb because anti-bonding effect is stronger than bonding effect.
22. Sigma (σ) molecular orbitals: A molecular orbital which is formed from the overlap of two s atomic orbitlas or head to head overlap of one s and p-atomic orbitals or head to head overlap of two p-atomic orbitals, is known as sigma molecular orbital.
23. pi (π) molecular orbitals: A molecular orbital which is formed by lateral overlap of two parallel p-orbitals is known as pi(π) molecular orbital.
24. Conditions for the Combination of Atomic Orbitals: The linear combination of atomic orbitals takes place only if the following conditions are satisfied :
(a) The combining atomic orbitals must have same or nearly same energy.
(b) The combining atomic orbitals must have the same symmetry about the molecular axis. By convention , z-axis is taken as the molecular axis.
(c) The combining atomic orbitals must overlap to the maximum extent. Greater the extent of overlapping, the greater will be electron density between the nuclei of a molecular orbital.
25. Energy level Diagrams for Molecular Orbitals: The increasing order of energies of various molecular orbitals for O2 and F2is given below.
* * * * *
σ1s < σ1s < σ2s <σ2pz < π2px = π2py <π2px =π2py < σ2pz
However, this sequence of energy levels of molecular orbitals is not correct for remaining molecules Li2, Be2, B2, C2, N2. For instance, it has been observed experimentally that for molecules such as B2, C2, N2 etc., the increasing order of energies of various molecular orbitals is
* * * * *
σ1s < σ1s < σ2s <σ2pz < π2px = π2py <π2px =π2py < σ2pz
The important characteristic feature of this order is that the energy of σ2pz molecular orbital is higher than that of π2px and π2py molecular orbitals in these molecules.