Structure of Atom Class 11 Notes are accessible in PDF format to assist students with reiterating the recently studied lessons before CBSE Board Exam. Thus, Chapter 2 Chemistry Class 11 Notes are prepared by our expert teachers who have long periods of teaching experience.
Structure of Atom Class 11 Notes PDF, is one of the most favored revision notes for students thinking that they are powerful to the point of getting higher marks in an Exam.
Chapter 2 Chemistry Class 11 Notes is assimilated with appropriate diagrams and compacted in such a way to make certain every detail is covered. You can easily download the Structure of Atom Class 11 Notes PDF to improve your preparation before taking the board exam.
Please refer to the Structure of Atom Revision Notes given below. These revision notes have been designed as per the latest NCERT, CBSE and KVS books issued for the current academic year. Students will be able to understand the entire chapter in your class 11th Chemistry book. We have provided chapter wise Notes for Class 11 Chemistry as per the latest examination pattern.
Revision Notes Chapter 2 Structure of Atom
Students of Class 11 Chemistry will be able to revise the entire chapter and also learn all important concepts based on the topic wise notes given below. Our best teachers for Grade 11 have prepared these to help you get better marks in upcoming examinations. These revision notes cover all important topics given in this chapter.
• Atom is the smallest indivisible particle of the matter. Atom is made of electron, proton and neutrons.

• Electrons were discovered using cathode ray discharge tube experiment.
• Nucleus was discovered by Rutherford in 1911.
Cathode ray discharge tube experiment: A cathode ray discharge tube made of glass is taken with two electrodes. At very low pressure and high voltage, current starts flowing through a stream of particles moving in the tube from cathode to anode. These rays were called cathode rays. When a perforatedanode was taken, the cathode rays struck the other end of the glass tube atthe fluorescent coating and a bright spot on the coating was developed
Results:
a. Cathode rays consist of negatively charged electrons.
b. Cathode rays themselves are not visible but their behavior can be observed with help of fluorescent or phosphorescent materials.
c. In absence of electrical or magnetic field cathode rays travel in straight lines
d. In presence of electrical or magnetic field, behaviour of cathode rays is similar to that shown by electrons
e. The characteristics of the cathode rays do not depend upon the material of the electrodes and the nature of the gas present in the cathode ray tube.
• Charge to mass ratio of an electron was determined by Thomson. The charge to mass ratio of an electron as 1.758820 x 1011 C kg-1
• Charge on an electron was determined by R A Millikan by using an oil drop experiment. The value of the charge on an electron is -1.6 x 10-19C.
• The mass on an electron was determined by combining the results of Thomson’s experiment and Millikan’s oil drop experiment. The mass of an electron was determined to be 9.1094 x 10-31kg.
• Discovery of protons and canal rays: Modified cathode ray tube experiment was carried out which led to the discovery of protons.
Characteristics of positively charged particles:
a. Charge to mass ratio of particles depends on gas from which these originate
b. The positively charged particles depend upon the nature of gas present in the cathode ray discharge tube
c. Some of the positively charged particles carry a multiple of fundamental of electrical charge.
d. Behaviour of positively charged particles in electrical or magnetic field is opposite to that observed for cathode rays
• Neutrons were discovered by James Chadwick by bombarding a thin sheet of beryllium by α- particles. They are electrically neutral particles having a mass slightly greater than that of the protons.
• Atomic number (Z) : the number of protons present in the nucleus (Moseley1913).
• Mass Number (A) :Sum of the number of protons and neutrons present in thenucleus.
• Thomson model of an atom: This model proposed that atom is considered asa uniform positively charged sphere and electrons are embedded in it.An important feature of Thomson model of an atom was that mass of atom isconsidered to be evenly spread over the atom.Thomson model of atom is also called as Plum pudding, raisin pudding orwatermelon modelThomson model of atom was discarded because it could not explain certainexperimental results like the scattering of α- particles by thin metal foils.
Observations from α- particles scattering experiment by Rutherford:
a. Most of the α- particles passed through gold foil un deflected
b. A small fraction of α- particles got deflected through small angles
c. Very few α- particles did not pass through foil but suffered large deflection nearly180o
Conclusions Rutherford drew from α- particles scattering experiment:
a. Since most of the α-particles passed through foil undeflected, it means most of the space in atom is empty
b. Since some of the α-particles are deflected to certain angles, it means that there is positively mass present in atom
c. Since only some of the α-particles suffered large deflections, the positively charged mass must be occupying very small space
d. Strong deflections or even bouncing back of α-particles from metal foil were due to direct collision with positively charged mass in atom
Rutherford’s model of atom: This model explained that atom consists ofnucleus which is concentrated in a very small volume. The nucleus comprisesof protons and neutrons. The electrons revolve around the nucleus in fixedorbits. Electrons and nucleus are held together by electrostatic forces of attraction.
Drawbacks of Rutherford’s model of atom:
a. According to Rutherford’s model of atom, electrons which are negatively charged particles revolve around the nucleus in fixed orbits. Thus,
b. theelectrons undergo acceleration. According to electromagnetic theory of Maxwell, a charged particle undergoing acceleration should emitelectromagnetic radiation. Thus, an electron in an orbit should emitradiation. Thus, the orbit should shrink. But this does not happen.
c. The model does not give any information about how electrons aredistributed around nucleus and what are energies of these electrons
Isotopes: These are the atoms of the same element having the same atomicnumber but different mass number.e g 1H1,1H2,1H3
Isobars: Isobars are the atoms of different elements having the same massnumber but different atomic number.e g 18Ar40 , 20Ca40
Isoelectronic species: These are those species which have the same nuwithelectrical and magnetic fields are called electromagnetic radiations. When anelectrically charged particle moves under acceleration, alternating electricaland magnetic fields are produced and transmitted. These fields aretransmitted in the form of waves. These waves are called electromagnetic waves or electromagnetic radiations.
Properties of electromagnetic radiations:
a. Oscillating electric and magnetic field are produced by oscillating charged particles. These fields are perpendicular to each other and both areperpendicular to the direction of propagation of the wave.
b. They do not need a medium to travel. That means they can even travel in vacuum.
Characteristics of electromagnetic radiations:
a. Wavelength: It may be defined as the distance between two neighbouring crests or troughs of wave as shown. It is denoted by λ.
b. Frequency (ν): It may be defined as the number of waves which passthrough a particular point in one second.
c. Velocity (v): It is defined as the distance travelled by a wave in onesecond. In vacuum all types of electromagnetic radiations travel with thesame velocity. Its value is 3 X108m sec-1. It is denoted by v
d. Wave number: Wave number is defined as the number of wavelengths per unit length.
Velocity = frequency x wavelength c = νλ
Planck’s Quantum Theory-
a.The radiant energy is emitted or absorbed not continuously but discontinuously in the form of small discrete packets of energy called ‘quantum’. In case of light , the quantum of energy is called a ‘photon’
b. The energy of each quantum is directly proportional to the frequency of the radiation, i.e. E αυ or E= hυ where h= Planck’s constant = 6.626 x 10-27 Js
c. Energy is always emitted or absorbed as integral multiple of this quantum. E=nhυ Where n=1,2,3,4,…..
• Black body: An ideal body, which emits and absorbs all frequencies, is called a black body. The radiation emitted by such a body is called black bodyradiation.
• Photoelectric effect: The phenomenon of ejection of electrons from thesurface of metal when light of suitable frequency strikes it is calledphotoelectric effect. The ejected electrons are called photoelectrons.
• Experimental results observed for the experiment of Photoelectric effecto When beam of light falls on a. metal surface electrons are ejected immediately.
b. Number of electrons ejected is proportional to intensity or brightness of light
c.Threshold frequency (vo): For each metal there is a characteristicminimum frequency below which photoelectric effect is not observed. Thisis called threshold frequency.
d. If frequency of light is less than the threshold frequency there is noejection of electrons no matter how long it falls on surface or how high isits intensity.
• Photoelectric work function (Wo): The minimum energy required to eject electrons is called photoelectric work function.Wo= hvo
• Energy of the ejected electrons :

• Dual behavior of electromagnetic radiation- The light possesses both particle and wave like properties, i.e., light has dual behavior . whenever radiation interacts with matter, it displays particle like properties.(Black body radiation and photoelectric effect) Wave like properties are exhibited when it propagates(interference an diffraction)
• When a white light is passed through a prism, it splits into a series ofcoloured bands known as spectrum.
• Spectrum is of two types: continuous and line spectrum
a. The spectrum which consists of all the wavelengths is called continuous spectrum.
b. A spectrum in which only specific wavelengths are present is known as a line spectrum. It has bright lines with dark spaces between them.
• Electromagnetic spectrum is a continuous spectrum. It consists of a range of electromagnetic radiations arranged in the order of increasing wavelengths ordecreasing frequencies. It extends from radio waves to gamma rays.
• Spectrum is also classified as emission and line spectrum.
a. Emission spectrum: The spectrum of radiationemitted by a substance that has absorbed energy is called an emissionspectrum.
b. Absorption spectrum is the spectrum obtained when radiation is passed through a sample of material. The sample absorbs radiation of certain wave lengths. The wave lengths which are absorbed are missing and comeas dark lines.
• The study of emission or absorption spectra is referred as spectroscopy.
• Spectral Lines for atomic hydrogen

• Rydberg equation
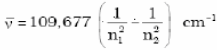
R = Rydberg’s constant = 109677 cm-1
• Bohr’s model for hydrogen atom:
a. An electron in the hydrogen atom can move around the nucleus in a circular path of fixed radius and energy. These paths are called orbits orenergy levels. These orbits are arranged concentrically around the nucleus.
b. As long as an electron remains in a particular orbit, it does not lose or gain energy and its energy remains constant.
c. When transition occurs between two stationary states that differ inenergy, the frequency of the radiation absorbed or emitted can be calculated

d. An electron can move only in those orbits for which its angular momentum is an integral multiple of h/2π
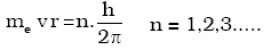
• The radius of the nth orbit is given byrn =52.9 pm x n2 /Z
• energy of electron in nth orbit is :

Limitations of Bohr’s model of atom:
a. Bohr’s model failed to account for the finer details of the hydrogen spectrum.
b. Bohr’s model was also unable to explain spectrum of atoms containing more than one electron.
Dual behavior of matter: de Broglie proposed that matter exhibits dualbehavior i.e. matter shows both particle and wave nature. de Broglie’s relation is

Heisenberg’s uncertainty principle: It states that it is impossible to determine simultaneously, the exact position and exact momentum (or velocity) of an electron.The product of their uncertainties is always equal to or greater than h/4π.

• Heisenberg’s uncertainty principle rules out the existence of definite pathsor trajectories of electrons and other similar particles
• Failure of Bohr’s model:
a. It ignores the dual behavior of matter.
b. It contradicts Heisenberg’s uncertainty principle.
• Classical mechanics is based on Newton’s laws of motion. It successfullydescribes the motion of macroscopic particles but fails in the case ofmicroscopic particles.
Reason: Classical mechanics ignores the concept of dual behaviour of matter especially for sub-atomic particles and the Heisenberg’s uncertainty principle.
• Quantum mechanics is a theoretical science that deals with the study of the motions of the microscopic objects that have both observable wave like andparticle like properties.
• Quantum mechanics is based on a fundamental equation which is calledSchrodinger equation.
• Schrodinger’s equation: For a system (such as an atom or a molecule whose energy does not change with time) the Schrödinger equation is written as:

• When Schrödinger equation is solved for hydrogen atom, the solution gives the possible energy levels the electron can occupy and the corresponding wave function(s) of the electron associated with each energy level.Out of the
possible values, only certain solutions are permitted. Each permitted solution is highly significant as it corresponds to a definite energystate. Thus, we can say that energy is quantized.
• ψ gives us the amplitude of wave. The value of ψhas no physicalsignificance.
• Ψ2 gives us the region in which the probability of finding an electron is maximum. It is called probability density.
Orbital: The region of space around the nucleus where the probability offinding an electron is maximum is called an orbital.
Quantum numbers: There are a set of four quantum numbers which specify the energy, size, shape and orientation of an orbital. To specify an orbital only three quantum numbers are required while to specify an electron all four quantum numbers are required.
Principal quantum number (n):It identifies shell, determines sizes and energy of orbitals

Azimuthal quantum number (l): Azimuthal quantum number. ‘l’ is also known as orbital angular momentum or subsidiary quantum number. l. It identifies sub-shell, determines the shape of orbitals, energy of orbitals in multi-electron atoms along with principal quantum number and orbital angular momentum, i.e.,

The number of orbitals in a subshell = 2l + 1. For a given value of n, it can have n values ranging from 0 to n-1. Total number of subshells in a particular shell is equal to the value of n.
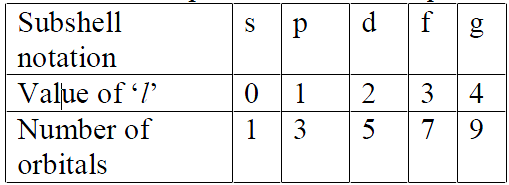
Magnetic quantum number or Magnetic orbital quantum number (ml):
It gives information about the spatial orientation of the orbital with respect to standard set of co-ordinate axis.For any sub-shell (defined by ‘l’ value) 2l+1 values of ml are possible.For each value of l, ml = – l, – (l –1), – (l–2)… 0,1… (l – 2), (l–1), l
Electron spin quantum number (ms): It refers to orientation of the spin of the electron. It can have two values +1/2 and -1/2. +1/2 identifies the clock wises pin and -1/2 identifies the anti- clockwise spin
• The region where this probability density function reduces to zero is called nodal surfaces or simply nodes.
Radial nodes: Radial nodes occur when the probability density of wave functionfor the electron is zero on a spherical surface of a particular radius. Numberof radial nodes = n – l – 1
Angular nodes: Angular nodes occur when the probability density wavefunction for the electron is zero along the directions specified by a particularangle. Number of angular nodes = l
• Total number of nodes = n – 1
Degenerate orbitals: Orbitals having the same energy are called degenerateorbitals.
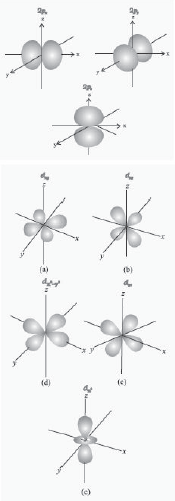
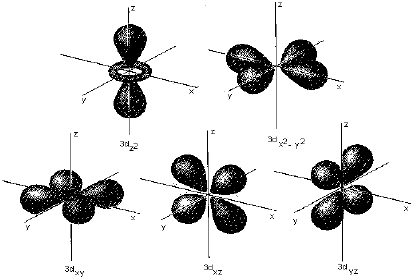
Shape of p and d-orbitals
Shielding effect or screening effect: Due to the presence of electrons in thei nner shells, the electron in the outer shell will not experience the full positivecharge on the nucleus. So, due to the screening effect, the net positive charge experienced by theelectron from the nucleus is lowered and is known as effective nuclear charge. Effective nuclear charge experienced by the orbital decreases with increase of azimuthal quantum number (l).
Aufbau Principle: In the ground state of the atoms, the orbitals are filled inorder of their increasing energies
• n+l rule-Orbitals with lower value of (n+l) have lower energy. If two orbitals have the same value of (n+l) then orbital with lower value of nwill have lower energy.
• The order in which the orbitals are filled isas follows:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 4f, 5d, 6p, 7s…
Pauli Exclusion Principle: No two electrons in an atom can have the same set of four quantum numbers. Only two electrons may exist in the same orbitaland these electrons must have opposite spin.
Hund’s rule of maximum multiplicity: Pairing of electrons in the orbitalsbelonging to the same subshell (p, d or f) does not take place until eachorbital belonging to that subshell has got one electron each i.e., it is singly occupied.
Electronic configuration of atoms: Arrangement of electrons in different orbitals of an atom. The electronic configuration of differentatoms can be represented in two ways.
a. sapbdc…… notation.
b. Orbital diagram:, each orbital of the subshell is represented by a box and the electron is represented by an arrow (↑) a positive spin or an arrow (↓) a negative spin.
Stability of completely filled and half filled subshells:
a. Symmetrical distribution of electrons- the completely filled or half filled sub-shells have symmetrical distribution of electrons in them and are more stable.
b. Exchange energy-The two or more electrons with the same spin present in the degenerate orbitals of a sub-shell can exchange their position and the energy released due to this exchange is called exchange energy. The number of exchanges is maximum when the subshell is either half filled or completely filled. As a result the exchange energy is maximum and so is the stability.
Important Points
1. The word ‘atom‘ has been derived from the Greek word ‘a-tomio‘ which means ‘uncutableor‘ ‘non-divisible‘.
2. J. J. Thomson, in 1898, proposed that an atom possesses a spherical shape (radius approximately 10–10 m) in which the positive charge is uniformly distributed. The electrons are embedded into it in such a manner as to give the most stable electrostatic arrangement Many different names are given to this model, for example, plum pudding, raisin pudding or watermelon.
3. Rutherford’s Nuclear Model of Atom:
a) Most of the space in the atom is empty as most of the a–particles passed through the foil undeflected.
b) A few positively charged a– particles were deflected. The deflection must be due to enormous repulsive force showing that the positive charge of the atom is not spread throughout the atom as Thomson had presumed. The positive charge has to be concentrated in a very small volume that repelled and deflected the positively charged a– particles.
c) Calculations by Rutherford showed that the volume occupied by the nucleus is negligibly small as compared to the total volume of the atom. The radius of the atom is about 10–10 m, while that of nucleus is 10–15 m.
d) On the basis of above observations and conclusions, Rutherford proposed the nuclear model of atom (after the discovery of protons). According to this model :
(i) The positive charge and most of the mass of the atom was densely concentrated in extremely small region. This very small portion of the atom was called nucleus by Rutherford.
(ii) The nucleus is surrounded by electrons that move around the nucleus with a very high speed in circular paths called orbits. Thus, Rutherford‘s model of atom resembles the solar system in which the nucleus plays the role of sun and the electrons that of revolving planets.
(iii) Electrons and the nucleus are held together by electrostatic forces of attraction.
4. The number of protons present in the nucleus is equal to atomic number (Z).the nucleus is equal
to atomic number (Z ).i.e.
Atomic number (Z) = number of protons in the nucleus of an atom = number of electronsin a nuetral atom
5. Protons and neutrons present in the nucleus are collectively known as nucleons. The total number of nucleons is termed as mass number (A) of the atom.mass number (A) = number of protons (Z) + number of neutrons (n)
6. Isobars are the atoms with same mass number but different atomic number for example, 6C14and
7N14. On the other hand, atoms with identical atomic number but different atomic mass number are known as Isotopes. e.g. 6C14 6C13 6C12 & 17Cl35 , 17Cl37
7. Drawbacks of Rutherford Model According to the electromagnetic theory of Maxwell, charged particles when accelerated should emit electromagnetic radiation (This feature does not exist for planets since they are uncharged). Therefore, an electron in an orbit will emit radiation, the energy carried by radiation comes from electronic motion. The orbit will thus continue to shrink. Calculations show that it should take an electron only 10–8 s to spiral into the nucleus . But this does not happen. Thus, the Rutherford model cannot explain the stability of an atom.
8. The frequency (ν ), wavelength (λ) and velocity of light (c) are related by the equation (2.5). c = ν λ The other commonly used quantity specially in spectroscopy, is the wavenumber (ν- ). It is defined as the number of wavelengths per unit length. Its units are reciprocal of wavelength unit, i.e., m–1.
9. H. Hertz performed a very interesting experiment in which electrons (or electric current) were ejected when certain metals (for example potassium, rubidium, caesium etc.) were exposed to a beam of light . The phenomenon is called Photoelectric effect. For photoelectric effect : hv = hv° + 1/2 mv2
10. Planck’s quantum theory.
(i) The energy is radiated or absorbed by a body not continuously but discontinuously in form of small packets.
(ii) Each packet is called quantum. In case of light, the quantum is called ‘photon‘. The energy of quantum is directly proportional to the frequency (v) of the radiation. E αv E = hv, Where ‘h’ is Planck‘s constant. Its value is 6.625 × 10-34 Joule second.
11. The spectrum of radiation emitted by a substance that has absorbed energy is called an emission spectrum. Atoms, molecules or ions that have absorbed radiation are said to be ―excited‖. To produce an emission spectrum, energy is supplied to a sample by heating it or irradiating it and the wavelength (or frequency) of the radiation emitted, as the sample gives up the absorbed energy, is recorded.
12. An absorption spectrum is like the photographic negative of an emission spectrum. A continuum of radiation is passed through a sample which absorbs radiation of certain wavelengths.
13. Line Spectrum of Hydrogen: When an electric discharge is passed through gaseous hydrogen, the H2 molecules dissociate and the energetically excited hydrogen atoms produced emit electromagnetic radiation of discrete frequencies. The hydrogen spectrum consists of several series of lines named after their discoverers.

The Swedish spectroscopist, Johannes Rydberg, noted that all series of lines in the hydrogen spectrum

could be described by the following expression :
14. Bohr’s Model For Hydrogen Atom
a) The electron in the hydrogen atom can move around the nucleus in a circular path of fixed radius and energy. These paths are called orbits, stationary states or allowed energy states. These orbits are arranged concentrically around the nucleus.
b) An electron can move only in those orbits for which its angular momentum is integral multiple of h/2Π that is why only certain fixed orbits are allowed.The angular momentum of an electron in a given stationary state can be expressed as in equation

c) The energy of an electron in the orbit does not change with time. However, the electron will move from a lower stationary state to a higher stationary state when required amount of energy is absorbed by the electron or energy is emitted when electron moves from higher stationary state to lower stationary state . The energy change does not take place in a continuous manner.
d) The frequency of radiation absorbed or emitted when transition occurs between two stationary states that differ in energy by ΔE, is given by :

15. Bohr‘s theory can also be applied to the ions containing only one electron, similar to that present in hydrogen atom. For example, He+ Li2+, Be3+ and so on. The energies of the stationary states associated with these kinds of ions (also known as hydrogen like species) are given by the

16. Limitations of Bohr’s Model: It fails to account for the finer details (doublet, that is two closely spaced lines) of the hydrogen atom spectrum observed by using sophisticated spectroscopic techniques. This model is also unable to explain the spectrum of atoms other than hydrogen, for example, helium atom which possesses only two electrons. Further, Bohr‘s theory was also unable to explain the splitting of spectral lines in the presence of magnetic field (Zeeman effect) or an electric field (Stark effect).
17. Dual Behaviour of Matter: The French physicist, de Broglie in 1924 proposed that matter, like radiation, should also exhibit dual behaviour i.e., both particle and wavelike properties.
18. The de Broglie relation. :de Broglie relation state that the wavelength associated with a moving object or an electron is inversely proportional to the momentum of the particle λ= h/ mv=h/p where p is the momentum of particle = mv.
19. Heisenberg’s Uncertainty Principle. It is not possible to determine the position and velocity simultaneously for a sub-atomic particle like electron at any given instant to an arbitary degree of precision. Consequently, it is not possible to talk of path of the electron in which it moves. If Δ x‘ is uncertainty in position and Δ P‘ is uncertainty in momentum then ΔxΔp>h/4Π
20. Orbital. It is a region or space where there is maximum probability of getting electron.
21. Quantum numbers. They are used to get complete information about electron, i.e., location, energy, spin, etc. These quantum numbers also help to designate the electron present in an orbital.
22. Principal quantum number. It specifies the location and energy of an electron. It is measure of the effective volume of the electron cloud. It is denoted by n‘. Its possible values are 1, 2, 3,4 …..
23. Angular momentum quantum number. It is also called azimuthal quantum number‘. It determines the shape of the orbital. It is denoted by l‘. The permitted values of l‘ are 0, 1, 2, etc., upto n–1. For a given value of n, l = 0 to n – 1 . e.g., if value of n is 4, l can have values 0, 1, 2, 3. It determines angular momentum

24. Magnetic quantum number. It is denoted by m‘ and its value depends on value of l‘ since magnetism is due to angular momentum. It determines the magnetic orientation of an orbital, i.e., the direction of orbital relative to magnetic field in which it is placed. Its permitted values are – l to + l including zero, e.g., when l = 1, then m = -1, 0, +1. It has total number of values equal to 2l + 1.
25. Spin quantum number. It indicates, the direction in which electron revolves. Spin is magnetic property and is also quantized. It has two permitted values + ½ or – ½. The spin angular momentum of an electron is constant and cannot be changed.
26. (n+l) rule: The relative order of energies of various sub-shells in a multi-electron atom can be predicted with the help of (n+l) rule (also called Bohr-Bury rule)According to this rule a subshell with lower values of (n+l) has lower energy.In case two sub-shell has equal value of (n+l), the sub-shell with lower value of n has lower energy
27. Pauli’s Exclusion Principle. No two electrons in an atom can have all the four quantum numbers same. It can also be stated as – An orbital can have maximum two electrons and they must be of opposite spin quantum numbers.
28. Aufbau principle. Electrons are filled in the various orbitals in the increasing order of their energies, i.e., orbital having lowest energy will be filled first and the orbital having highest energy will be filled last.Increasing energy ofatomic orbitals for multi-electron atoms 1s < 2s < 2p < 3s <3p < 4s <3d < 4p <5s < 4d <5p < 6s < 4f < 5d < 6p < 7s
29. Hund’s rule of maximum multiplicity. No electron pairing takes place in p, d and f orbitals until each orbital in the given sub-shell contains one electron, e.g., N (7) has electronic configuration 1s2 2s2 2p1 2p1 2p1 according to Hund‘s rule and not 1s2 2s2 2p1 2p1
30. The valence electronic configurations of Cr and Cu, therefore, are 3d5 4s1 and 3d104s1 respectively and not 3d4 4s2 and 3d94s2. It has been found that there is extra stability(Stability of Completely Filled and Half Filled Subshells) associated with these electronic configurations.
31. Three orbitals of 2p subshell (2px, 2py, and 2pz orbitals).

32. Five orbitals of 3d subshell (3dxy, 3dyz, 3dzx 3dx 2-y2and 3dx2 orbitals).

At Assignmentbag, we intend to give information to the students in an imaginative way. It is the explanation that we are reliably attempting to carry out more new er methods for learning through our facility.
Our teachers are specialists in their specific subjects, and with their long period of teacher experience, they have customized these revision notes with care. We give the most standard Structure of Atom Class 11 Notes in PDF format to help students to make their preparation better before taking the board exam. The use of basic language and plain portrayal of information permits students to review the detail quicker.


