Please refer to the Equilibrium Revision Notes given below. These revision notes have been designed as per the latest NCERT, CBSE and KVS books issued for the current academic year. Students will be able to understand the entire chapter in your class 11th Chemistry book. We have provided chapter wise Notes for Class 11 Chemistry as per the latest examination pattern.
Revision Notes Chapter 7 Equilibrium
Students of Class 11 Chemistry will be able to revise the entire chapter and also learn all important concepts based on the topic wise notes given below. Our best teachers for Grade 11 have prepared these to help you get better marks in upcoming examinations. These revision notes cover all important topics given in this chapter.
- Equilibrium state: When rate of formation of a product in a process is in competition with rate of formation of reactants, the state is then named as “Equilibrium state” .
- Equilibrium in physical processes: solid ⇌ liquid ⇌ gas
H2O(s )⇌ H2O(l) ⇌ H2O(vap) - Law of chemical equilibrium: At a given temperature, the product of concentrations of the reaction products raised to the respective stoichiometriccoefficient in the balanced chemical equation divided by the product of concentrations of the reactants raised totheir individual stoichiometric coefficients has a constant value. This is known as the Equilibrium Law or Law of Chemical Equilibrium.
aA +bB⇌cC + dD
Kc =[C]c [D]d/[A]a [B]b
Chemical equation Equilibriumconstant
aA + b B⇌c C + D K
cC + d D⇌a A + b B K′c=(1/Kc)
na A + nb B ⇌ncC + ndD K′″c= (Kcn)
Concentrations or partial pressure of pure solids or liquids do not appear in the expression of the equilibrium constant. In the reaction,
Ag2O(s) + 2HNO3(aq) ⇌2AgNO3(aq) +H2O(l)kc = (AgNO3)2 / (HNO3)2 - If Qc >Kc, the reaction will proceed in the direction of reactants (reverse reaction).If Qc <Kc, the reaction will proceed in the direction of the products (forward reaction)
- Kp is equilibrium constant in terms of partial pressure of gaseous reactants and products.
- Kc is equilibrium constant in terms of molar concentration of gaseous reactants and products.
- Kp =Kc (RT)Δn here R is gas constant, T is temperature at which the process is carried out &Δn is no. of moles of gaseous product minus no. of moles of gaseous reactants.
- If Kc> 103; Kc is very high i.e. the reaction proceeds nearly to completion.
- If Kc<103; Kc is very small i.e. the reaction proceeds rarely.
- If Kcis ranging in the range of 103to 10-3; i.e. reactants and products are just in equilibrium.
- ΔG° = – RT lnK or ΔG°= – 2.303RT log K
- Factors affecting equilibrium constant:- temperature, pressure, catalyst and molar concentration of reactants and products.
- Le Chatelier’s principle:- It states that a change in any of the factors that determine the equilibrium conditions of a system will cause the system to change in such a manner so as to reduce or to counter act the effect of the change.
- Arrhenius acids are the substances that ionize in water to form H+.
- Arrhenius bases are the substances that ionize in water to form OH–.
- Lewis acids are lone pair (of e-) accepters while Lewis bases are lone pair donators.
- Proton donor are acids while proton accepters are bases(Bronsted-Lowry concept).
- The acid-base pair that differs only by one proton is called a conjugate acid base pair. If Brönsted acid is a strong acid then its conjugate base is a weak base and viceversa.
- Ionic product of water.Kw = [H+][OH–]
- pH = -log [H+] ; here[H+] is molar concentration of hydrogen ion.
- pH + pOH =14
- pKa + pKb =14
- Ka x Kb = Kw = ionic product of water=1 x 10-14
- Buffer solution :The solutions which resist change in pH on dilution or with the addition of small amounts of acid or alkali are called Buffer Solutions.
- common ion effect: It can be defined as a shift in equilibrium on adding a substance that provides more of an ionic species already present in the dissociation equilibrium.
- Hydrolysis of Salts: process of interaction between water and cations/anions or both of salts is called hydrolysis.
- The cations (e.g., Na+, K+,Ca2+, Ba2+, etc.) of strong bases and anions(e.g., Cl–, Br–, NO3–, ClO4– etc.) of strong acids simply get hydrated but do not hydrolyse, and therefore the solutions of salts formed from strong acids and bases are neutral i.e., their pH is 7.
- Salts of weak acid and strong base e.g.,CH3COONa are basic in nature.
- Salts of strong acid and weak base e.g.,NH4Cl, are acidic
- Salts of weak acid and weak base, e.g.,CH3COONH4. The pH is determined by the formula pH = 7 + ½ (pKa – pKb)
- Solubility product- product of the molar concentrations of the ions in a saturated solution,each concentration term raised to the power equal to the no. of ions produced.
Important Points
1. Equilibrium represents the state of a process in which the properties like temperature, pressure etc do not show any change with the passage of time
2. Chemical equilibrium: When the rates of the forward and reverse reactions become equal, the concentrations of the reactants and the products remain constant. This is the stage of chemical equilibrium. This equilibrium is dynamic in nature as it consists of a forward reaction in which the reactants give products and reverse reaction in which products gives the original reactants. Equilibrium is possible only in a closed system at a given temperature. A mixture of reactants and products in the equilibrium state is called an equilibrium mixture.
3. In a Homogeneous system, all the reactants and products are in the same phase. For example, in the gaseous reaction, N2 (g) + 3H2(g)→2NH3(g), reactants and products are in the homogeneous phase.
4. Equilibrium in a system having more than one phase is called heterogeneous equilibrium. The equilibrium between water vapor and liquid water in a closed container is an example of heterogeneous equilibrium. H2O(l) → H2O(g)
5. Henry Law: It states that the mass of a gas dissolved in a given mass of a solvent at any temperature is proportional to the pressure of the gas above the solvent
6. Law of Chemical Equilibrium: It may be stated as, at a given temperature the ratio of product of equilibrium concentration of the products to that of the reactants with each concentration terms raised to power equal to the respective stoichiometric coefficient in the balanced chemical reaction has a constant value. This constant value is known as Equilibrium constant. For a general reaction of the type
aA + bB ⇔ cC + dD
Kc = [C]c[D]d /[A]a [B]b This expression is known as Law Of Chemical Equilibrium
7. Relationship between Kp and Kc: Kp = Kc(RT) Δn
8. Units of Equilibrium Constant: The value of equilibrium constant Kc can be calculated by substituting the concentration terms in mol/L and for Kp partial pressure is substituted in Pa, kPa, bar or atm. This results in units of equilibrium constant based on molarity or pressure, unless the exponents of both the numerator and denominator are same. For the reactions
(i)H2(g) + I2(g) → 2HI, Kc and Kp have no unit.
(ii)N2O4(g) → 2NO2 (g), Kc has unit mol/L and Kp has unit bar
9. Characteristics Of Equilibrium Constant
♦ Equilibrium constant is applicable only when concentrations of the reactants and products have attained their equilibrium state.
♦ The value of equilibrium constant is independent of initial concentrations of the reactants and products.
♦ Equilibrium constant is temperature dependent having one unique value for aparticular reaction represented by a balanced equation at a given temperature.
♦ The equilibrium constant for the reverse reaction is equal to the inverse of the equilibrium constant for the forward reaction.
♦ The equilibrium constant K for a reaction is related to the equilibrium constant of the corresponding reaction, whose equation is obtained by multiplying or dividing the equation for the original reaction by a small integer.
10. Applications of equilibrium constant :
♦ Predict the extent of a reaction on the basis of its magnitude.
♦ Predict the direction of the reaction
♦ Calculate equilibrium concentrations.
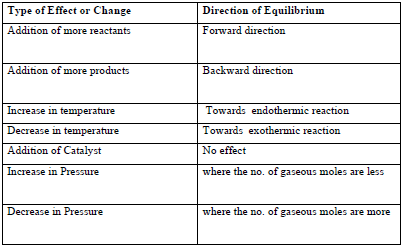
11. Le Chatelier’s Principle: It states that if a system in equilibrium is subjected to a change of concentration, temperature or pressure, the equilibrium shifts in a direction that tends to undo the effect of the change imposed.
♦ Effect of change of concentration: When the concentration of any of the reactants or products in a reaction at equilibrium is changed, the composition of the equilibrium mixture changes so as to minimize the effect of concentration change. For eg:- H2(g) + I2(g) ⇔ 2HI(g)
If H2 is added to the reaction mixture at equilibrium, the equilibrium of the reaction is disturbed. In order to restore it, the reaction proceeds in a direction whereas H2 is consumed i.e more of H2and I2 react to form HI and finally the equilibrium shifts in forward direction.
♦ Effect of change of pressure: When the pressure is increased the equilibrium hifts in the direction in which the number of moles of the gas decreases. Consider the reaction, CO (g) + 3H2 (g) ⇔ CH4 (g) + H2O (g) Here, 4 mol of gaseous reactants (CO + 3H2) become 2 mol of gaseous products (CH4 (g) + H2O ). so by Le Chatelier‘s principle. The increase in pressure will shift the equilibrium in the forward direction, a direction in which the number of moles of the gas or pressure decreases.
♦ Effect of change of Temperature: When a change in temperature occurs, the value of equilibrium constant changes. In general, the temperature dependence of the equilibrium constant depends on the sign of ΔH for the reaction. The equilibrium constant for an exothermic reaction (-ve ΔH) decreases as the temperature increases. The equilibrium constant for an endothermic reaction (+ve ΔH) increases as the temperature increases. When the Temperature is increased the equilibrium shifts in the direction in of endothermic reaction.
Consider a reaction N2(g) + 3H2(g) ⇔ 2NH3(g) ΔH = -92.38Kj/mol
According to Le Chatelier‘s principle, raising the temperature shifts the equilibrium to left (backward direction i.e direction of endothermic reaction) and decreases the equilibrium concentration of ammonia.
♦ Effect of Inert Gas Addition: If the volume is kept constant and an inert gas such as argon is added which does not take part in the reaction, the equilibrium remains undisturbed. It is because the addition of an inert gas at constant volume does not change the partial pressures or the molar concentrations of the substance involved in the reaction. The reaction quotient changes only if the added gas is a reactant or product involved in the reaction.
♦ Effect of a Catalyst: A catalyst increases the rate of the chemical reaction by making available a new low energy pathway for the conversion of reactants to products. It increases the rate of forward and reverse reactions that pass through the same transition state and does not affect equilibrium. Catalyst lowers the activation energy for the forward and reverse reactions by exactly the same amount. Catalyst does not affect the equilibrium composition of a reaction mixture. It does not appear in the balanced chemical equation or in the equilibrium constant expression.
Summary of Le Chatelier’s Principle