Please refer to Amines HOTs Class 12 Chemistry provided below with solutions. All HOTs for Class 12 Chemistry with answers provided below have been designed as per the latest syllabus and examination petter issued by CBSE, NCERT, KVS. Students of Standard 12 Chemistry should learn the solved HOTS for Class 12 Chemistry provided below to gain better marks in examinations.
Amines Class 12 Chemistry HOTs
Question. In the reaction,
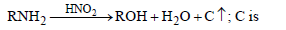
(a) NH3
(b) N2
(c) O2
(d) CO2
Answer
B
Question. Amines behave as
(a) lewis acids
(b) lewis bases
(c) aprotic acids
(d) amphoteric compounds
Answer
B
Question. What is the IUPAC name of the following compound ?
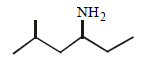
(a) 2-methyl-4-hexanamine
(b) 5-methyl-3-hexanamine
(c) 2-methyl-4-amino hexane
(d) 5-methyl-3-amino hexane
Answer
B
Question. Which of the following statement is correct ?
(a) Ammonia is more basic than methylamine.
(b) Methylamine is more basic than ammonia.
(c) Dimethylamine is less basic than methylamine.
(d) Dimethylamine is less basic than trimethylamine.
Answer
B
Question. Substitution of one alkyl group by replacing hydrogen of primary amines
(a) increases the base strength
(b) decreases the base strength
(c) remains the same
(d) None of the above
Answer
A
Question. For carbylamine reaction, we need hot alcoholic KOH and
(a) any primary amine and chloroform
(b) chloroform and silver powder
(c) a primary amine and an alkyl halide
(d) a monoalkylamine and trichloromethane.
Answer
A
Question. Propionamide on Hofmann degradation gives –
(a) methyl amine
(b) ethyl amine
(c) propyl amine
(d) ethyl cyanide
Answer
B
Question.The best reagent for converting 2 – phenylpropanamide into 2-phenylpropanamine is ___________.
(a) excess H2
(b) Br2in aqueous NaOH
(c) iodine in the presence of red phosphorus
(d) LiAlH4in ether
Answer
D
Question. The correct order of basicity of the following compounds

(a) B > A > C
(b) A > B > C
(c) C > A > B
(d) C > B > A
Answer
C
Question. Which of the following compounds is most basic?

Answer
B
Question. Amines play an important role in the survival of life.
Naturally they are found in
(a) proteins
(b) vitamins
(c) alkaloids
(d) All of these
Answer
D
Question. The correct decreasing order of basic strength of the following species is __________ H2O, NH3, OH–, NH2–
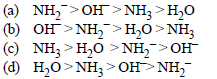
Answer
A
Question. Ethyl amine can be obtained by the
(a) Action of NH3 on ethyl iodide.
(b) Action of NH3 on ethyl alcohol.
(c) Both (a) and (b)
(d) Neither (a) nor (b)
Answer
C
Question. Which of the following factors affect the basic strength of amine?
(i) Inductive effect
(ii) Steric hinderance
(iii) Solvation effect
(iv) Solubility in organic solvents.
(a) (i) and (iv)
(b) (i), (ii) and (iii)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer
B
Question. Which of the following amines can be prepared by Gabriel method ?
(i) CH3CH2NH2
(ii) (CH3)2CHNH2
(iii) (CH3)3CNH2
(iv) C6H5NH2
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i), (ii) and (iii)
(d) (i) and (ii)
Answer
D
Question. Aliphatic amines are…..basic than NH3 but aromatic amines are……basic than NH3.
(a) more, less
(b) less, more
(c) both (a) and (b)
(d) None of these
Answer
A
Question. Aniline is used
(a) in crimping of wool
(b) in dyeing industry
(c) in making of glue
(d) in fast drying vanish
Answer
B
Question. In the ammonolysis of alkyl halides the halogen atom is replaced by an amino(–NH2) group which of the following represent the correct order of reactivity of halides with amines.
(a) RBr > RI > RCl
(b) RI > RCl > RBr
(c) RI > RBr > RCl
(d) RCl > RBr > RI
Answer
C
Question. Gabriel’s phthalimide synthesis is used for the preparation of
(a) Primary aromatic amines
(b) Secondary amines
(c) Primary aliphatic amines
(d) Tertiary amines
Answer
C
Question. Mark the correct statement
(a) Methylamine is slightly acidic
(b) Methylamine is less basic than ammonia
(c) Methylamine is a stronger base than ammonia
(d) Methylamine forms salts with alkalies.
Answer
C
Question. Which of the following compounds is the weakest Bronsted base?
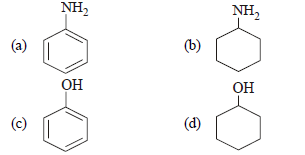
Answer
C
Question. Which statement is not true among the following?
(a) Amines are bases
(b) They turn red litmus blue
(c) Trimethyl amine is less basic than dimethyl amine
(d) Amines yield alcohols on aqueous hydrolysis.
Answer
D
Question.

Answer
A
Question. Aniline is less soluble in water than ethyl amine due to
(a) resonance stablization of benzene ring
(b) resonance stabilization of anilium ion
(c) more hydrophobic nature of C6H5 group than C2H5 group
(d) more hydrophobic nature of C6H5 group than C2H5 group
Answer
C
CRITICAL THINKING TYPE QUESTIONS
Question. What is the decreasing order of basicity of primary,secondary and tertiary ethylamines and NH3 ?
(a) NH3 > C2H5NH2 > (C2H5)2NH > (C2H5)3N
(b) (C2H5)3N > (C2H5)2NH > C2H5NH2 > NH3
(c) (C2H5)2NH > C2H5NH2 > (C2H5)3N > NH3
(d) (C2H5)2 NH > C2H5NH2 > (C2H5)3 NH > NH3
Answer
D
Question. Which of the following is the correct IUPAC name of the compound ?
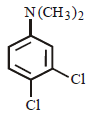
(a) 1, 2-dichloro-4-(N, N-dimethyl) aniline
(b) Dimethyl – (3, 4-dichlorophenyl) amine
(c) 3, 4-dichloro – N, N-dimethyl aniline
(d) N, N-dimethylamino – 3, 4-dichlorobenzene
Answer
C
Question.
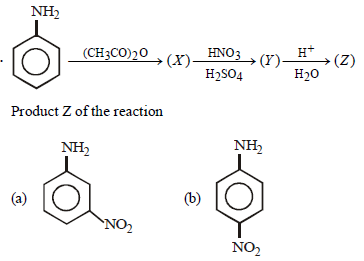

Answer
B
Question. A primary amine is formed by an amide on treatment with bromine and alkali. The primary amine has
(a) 1 carbon atom less than amide
(b) 1 carbon atom more than amide
(c) 1 hydrogen atom less than amide
(d) 1 hydrogen atom more than amide
Answer
A
Question. The most reactive amine towards dilute hydrochloric acid is _________ .
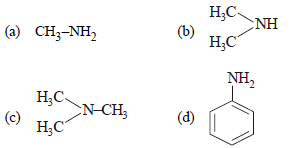
Answer
B
Question. Which one of the following is the strongest base in aqueous solution ?
(a) Methylamine
(b) Trimethylamine
(c) Aniline
(d) Dimethylamine
Answer
D
Question. The correct increasing order of basic strength for the following compounds is __________.
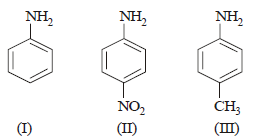
(a) II < III < I
(b) III < I < II
(c) III < II < I
(d) II < I < III
Answer
D
Question. The correct order of the increasing basicity of methyl amine, ammonia and aniline is
(a) methyl amine < aniline < ammonia
(b) aniline < ammonia < methyl amine
(c) aniline < methyl amine < ammonia
(d) ammonia < aniline < methyl amine
Answer
B
Question. The IUPAC name of diethyl isopropyl amine is
(a) N, N-diethylpropan-2-amine
(b) N, N-diethylpropan-1-amine
(c) N, N-diethylisopropylamine
(d) N, N-diethylaminopropane
Answer
A
Question. Arrange the following amines in the decreasing order of their basicity
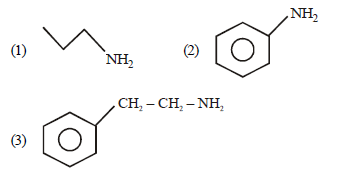
(a) 1 > 3 > 2
(b) 3 > 2 > 1
(c) 1 > 2 > 3
(d) 2 > 1 > 3
Answer
A
Question. Amine that cannot be prepared by Gabriel phthalimide synthesis is
(a) aniline
(b) benzylamine
(c) methylamine
(d) iso-butylamine
Answer
A
Question. A compound of molecular formulae C3H6N shows following characteristics
(i) Get dissolved in acidic medium.
(ii) Does not react with benzoylchloride
(iii) Does not give carbylamine test
(iv) Does not evolute nitrogen gas on reacting with HNO2 than structure of the compound is
(a) trimethylamine
(b) isopropylamine
(c) propylamine
(d) None of these
Answer
A
Question. Which of the statement is true regarding the basicity of the following two primary amines ?

(a) Both are equally basic because both are 1º amines
(b) I > II because it is an aromatic amine
(c) II > I because it is an aliphatic amine
(d) I < II because of difference in the nature of β-carbon
Answer
D
Question. In a reaction of aniline a coloured product C was obtained.

The structure of C would be :
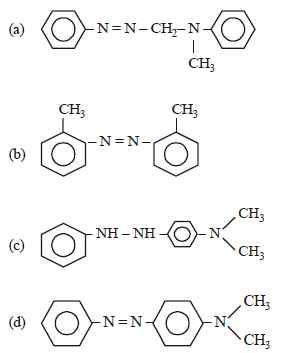
Answer
D
Question. Aniline when treated with conc. HNO3 gives
(a) p-Phenylenediamine
(b) m-Nitroaniline
(c) p-Benzoquinone
(d) Nitrobenzene
Answer
B
Question. Acetamide is treated with the following reagents separately.
Which one of these would yield methylamine?
(a) NaOH – Br2
(b) Sodalime
(c) Hot conc. H2SO4
(d) PCl5
Answer
A
Question. Which of the following compounds is most basic?
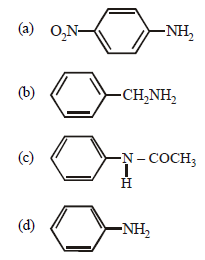
Answer
B
Question. IUPAC name of the following compound is
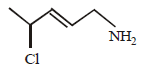
(a) 2-chloro pentanamine
(b) 4-chloro pentan-1-amine
(c) 4-chloro pent-2-en-1-amine
(d) 2-chloro pent-3-en-5-amine
Answer
C
Question. High basicity of Me2NH relative to Me3N is attributed to:
(a) effect of solvent
(b) inductive effect of Me
(c) shape of Me2NH
(d) shape of Me3N
Answer
A
Question. Towards electrophilic substitution, the most reactive will be
(a) Nitrobenzene
(b) Aniline
(c) Aniline hydrochloride
(d) N-Acetylaniline
Answer
B
Question. The correct order of decreasing basic character is

(a) II > I > III > IV
(b) IV > II > I > III
(c) IV > III > II > I
(d) IV > II > III > I
Answer
B
Question. Nitration of nitrobenzence is carried out than obtained product is reduced with Fe/HCl, product so formed on reaction with HNO2 and than with H2O, forms
(a) 1,3–dihydroxybenzene
(b) 3–nitrophenol
(c) 2– nitrophenol
(d) 1,2–dihydroxybenzene
Answer
B
Question.

Answer
C
Question.

The basicity order of I, II and III is –
(a) III > I > II
(b) I > II > III
(c) III > II > I
(d) II > III > I
Answer
A