Please refer to The d – and f – Block Elements Class 12 Chemistry Important Questions with solutions provided below. These questions and answers have been provided for Class 12 Chemistry based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these problem solutions as it will help them to gain more marks in examinations. We have provided Important Questions for Class 12 Chemistry for all chapters in your book. These Board exam questions have been designed by expert teachers of Standard 12.
Class 12 Chemistry Important Questions The d – and f – Block Elements
Very Short Answer Questions
Question. Account for the following :
Zn is not considered as a transition element.
Answer. In the electronic configuration of Zn, Cd and Hg the d-orbitals are completely filled in the ground state as well as in their common oxidation state. So, they are not regarded as transition metals.
Question. On what ground can you say that scandium (Z = 21) is a transition element but zinc (Z = 30) is not?
Answer. On the basis of incompletely filled d-orbitals :
Scandium (Z = 21), atom has incompletely filled d-orbitals (3d1) in its ground state, so it is regarded
as transition element. On the other hand zinc (Z = 30) atom has completely filled d-orbitals (3d10) in its ground state as well as most common oxidation state of +2.
Question. Complete the following equation :
2MnO–4 + 6H+ + 5NO–2 →
Answer. 2MnO–4 + 6H+ + 5NO2– → 2Mn2+ + 5NO–3 +3H2O
Question. Complete the following equation :
3MnO42– + 4H+ →
Answer. 3MnO2–4 + 4H+→ 2MnO–4 + MnO2 + 2H2O
Question. Complete the following equation :
MnO–4 + 8H+ + 5e– →
Answer. MnO–4 + 8H+ + 5e– → Mn2+ + 4H2O
Question. Complete the following chemical equations :
SO2 + MnO–4 + H2O →
Answer. 2MnO–4 + 5SO2 + 2H2O → 2Mn2+ + 5SO42– + 4H+
Question. Give reason :
Orange solution of potassium dichromate turns yellow on adding sodium hydroxide to it.
Answer. When the pH of the solution of potassium dichromate is decreased, the colour of the solution changes from yellow to orange due to the conversion of CrO42– ions into Cr2O72– ions
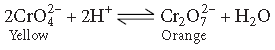
Question. Complete the following chemical equation :
Cr2O2–7(aq) + H2S(g) + H+(aq) →
Answer. Cr2O2–7(aq) + 3H2S(g) + 8H+(aq) → 2Cr3+(aq) + 7H2O(l) + 3S(s)
Question. Account for the following :
Zr and Hf have almost similar atomic radii.
Answer. Due to lanthanoid contraction the elements of 4d and 5d-series have similar atomic radii e.g.,
Zr = 145 pm and Hf = 144 pm.
Question. Name a member of the lanthanoid series which is well known to exhibit +2 oxidation state.
Answer. Europium (Eu) is well known to exhibit +2 oxidation state due to its half-fliled f orbital in +2 oxidation state.
Question. Give reason for the following :
The second and third transition series elements have almost similar atomic radii.
Answer. Ce(III) has outer configuration 4f15d06s0. It easily loses an electron to acquire the configuration 4f0 and forms Ce(IV). In fact this is the only (+IV) lanthanoid which exists in solution.
Question. Give reasons :
Actinoids show irregularities in their electronic configurations.
Answer. The irregularities in the electronic configurations of actinoids are due to extra stabilities of the f0 f7 and f14 orbitals.
Question. How would you account for the following:
Actinoid contraction is greater than lanthanoid contraction?
Answer. The actinoid contraction is more than lanthanoid contraction because 5f-electrons are more poorly shielding than 4f-electrons.
Short Answer Questions
Question. What are the transition elements? Write two characteristics of the transition elements.
Answer. Elements which have incompletely filled d-orbitals in their ground state or in any one of their oxidation states are called transition elements.
Characteristics of transition elements :
(i) They show variable oxidation states.
(ii) They exhibit catalytic properties.
Question. Write down the electronic configuration of
(a) Cr3+ (b) Cu+
(c) Co2+ (d) Mn2+
Answer.
(a) Cr3+ = 18[Ar] 3d3 (b) Cu+ = 18[Ar] 3d10
(c) Co2+ = 18[Ar] 3d7 (d) Mn2+ = 18[Ar] 3d5
Question. (i) Which metal in the first transition series (3d-series) exhibits +1 oxidation state most frequently and why?
(ii) Which of following cations are coloured in aqueous solutions and why?
Sc3+, V3+, Ti4+, Mn2+
(At. Nos. Sc = 21, V = 23, Ti = 22, Mn = 25)
Answer. (i) Copper exhibits +1 oxidation state in its compounds. Electronic configuration of Cu in the ground state is 3d10 4s1. So, Cu can easily lose 4s1 electron to give a stable 3d10 configuration. Thus it shows +1 oxidation state.
(ii) Only those ions will be coloured which have partially filled d-orbitals facilitating d-d transition.
Ions with d0 and d10 will be colourless. From electronic configuration of the ions, V3+(3d2) and Mn2+(3d5), are all coloured. Ti4+(3d0) and Sc3+(3d0) are colourless.
Question. How would you account for the following?
(i) Many of the transition elements are known to form interstitial compounds.
(ii) The metallic radii of the third (5d) series of transition metals are virtually the same as those of the corresponding group member of the second (4d) series.
Answer. (i) Transition metals form a large number of interstitial compounds because small atoms of certain non metallic elements (H, B, C, N, etc.) get trapped in voids or vacant spaces of lattices of the transition metals. As a result of filling up of the interstitial spaces such interstitial compounds are hard and rigid.
(ii) This is due to lanthanoid contraction
Question. How would you account for the following?
(i) With the same d-orbital configuration (d4) Cr2+ is a reducing agent while Mn3+ is an oxidising agent.
(ii) Most of the transition metal ions exhibit characteristic colours in aqueous solutions.
Answer. (i) E° values for the Cr3+/Cr2+ and Mn3+/Mn2+ couples are

Question. How is the variability in oxidation states of transition elements different from that of non-transition elements? Illustrate with examples.
Answer. The variability in oxidation states of transition metals is due to the incomplete filling of d-orbitals. Their oxidation states differ from each other by unity.
For example, Fe3+ and Fe2+, Cu2+ and Cu+, etc. In case of non transition elements the oxidation states normally differ by units of two. For example Pb2+ and Pb4+, Sn2+ and Sn4+, etc. It arises due to expansion of octet and inert pair effect.
Question. Describe the preparation of potassium permanganate. How does the acidified permanganate solution react with oxalic acid?
Write the ionic equations for the reaction.
Answer. Preparation of potassium permanganate : Potassium permanganate is prepared by the fusion of MnO2 (pyrolusite) with potassium hydroxide and an oxidising agent like KNO3 to form potassium manganate which disproportionates in a neutral or acidic solution to form permanganate.
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
3MnO42– + 4H+ → 2MnO–4 + MnO2 + 2H2O
or, 3K2MnO4 + 4HCl → 2KMnO4 + MnO2 + 2H2O+ 4KCl
2MnO2–4 + 16H+ + 5C2O2–4 → 2Mn2+ + 10CO2 + H2O
Question. With reference to structural variability and chemical reactivity, write the differences between lanthanoids and actinoids.
Answer. Structure : All the lanthanoids are silvery white soft metals. Hardness of Lanthanoids increases with increasing atomic number. The actinoid metals are all silvery in appearance but display a variety of structures. The structural variability is due to irregularities in metallic radii which are greater than that of lanthanoids.
Chemical reactivity : Earlier members of lanthanoid series are quite reactive similar to calcium but with increasing atomic number they behave more like aluminium. The actinoids are highly reactive in finely divided state.
Long Answer Questions
Question. Lokesh is a social worker. A milkman in the village has been complaining that a factory in his nearby area dumps chemical waste in his field which has become a major cause of decreasing productivity. Lokesh visited that place and found after analyis that the major waste was potassium permanganate which is being absorbed by the soil. He advised the factory people that they should treat potassium permanganate solution before dumping it into the drain.
Comment in brief
(i) About the value/s displayed by Lokesh.
(ii) Write balanced chemical equations for the two reactions showing oxidizing nature of potassium permanganate. (VBQ)
Answer. (i) The values, associated with Lokesh are alertness, care, responsibility and scientific knowledge.
(ii) (a) Oxidation of Oxalate ion into CO2
Question. The elements of 3d transition series are given as aSc Ti V Cr Mn Fe Co Ni Cu Zn
Answer the following :
(i) Write the element which shows maximum number of oxidation states. Given reason.
(ii) Which element has the highest melting point?
(iii) Which element shows only +3 oxidation state?
(iv) Which element is a strong oxidising agent in +3 oxidation state and why?
Answer. (i) Mn shows maximum no. of oxidation states from +2 to +7 because Mn has maximum number of unpaired electrons in 3d sub-shell.
(ii) Cr has maximum melting point, because it has 6 unpaired electrons in the valence shell, hence it has strong interatomic interaction.
(iii) Sc shows only +3 oxidation state because because after losing 3 electrons, it has noble gas electronic configuration.
(iv) Mn is strong oxidising agent in +3 oxidation state because change of Mn3+ to Mn2+ give stable half filled (d5) electronic configuration, E°(Mn3+/Mn2+) = 1.5 V.