Please refer to Assignments Class 12 Chemistry Alcohols, Phenols and Ethers Chapter 11 with solved questions and answers. We have provided Class 12 Chemistry Assignments for all chapters on our website. These problems and solutions for Chapter 11 Alcohols, Phenols and Ethers Class 12 Chemistry have been prepared as per the latest syllabus and books issued for the current academic year. Learn these solved important questions to get more marks in your class tests and examinations.
Alcohols, Phenols and Ethers Assignments Class 12 Chemistry
Question. Which of the following compounds will react with sodium hydroxide solution in water?
(A) C6H5OH
(B) C6H5CH2OH
(C) (CH3)3COH
(D) C2H5OH
Answer
C
Question. Williamson synthesis is used to obtain
(A) Primary alcohol
(B) Ether
(C) Aldehyde
(D) Ketone
Answer
B
Question. Phenol is less acidic than ______________.
(A) ethanol
(B) o-nitrophenol
(C) o-methylphenol
(D) o-methoxyphenol
Answer
B
Question. Which of the following species can act as the strongest base?
(A) QOH
(B) QOR
(C) QOC6H5
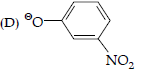
Answer
B
Question. How many alcohols with molecular formula C4H10O are chiral in nature?
(A) 1
(B) 2
(C) 3
(D) 4
Answer
A
Question. Which of the following compounds is aromatic alcohol?
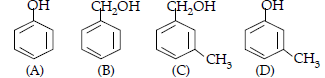
(A) A, B, C, D
(B) A, D
(C) B, C
(D) A
Answer
C
Question. IUPAC name for the given compound is
(A) 2-ethoxy-2-methylethane.
(B) 2-propoxypropane.
(C) 2-methyl-2-ethoxypropane
(D) None of the above
Answer
B
Question. Give IUPAC name of the compound given below.
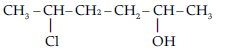
(A) 2-Chloro-5-hydroxyhexane
(B) 2-Hydroxy-5-chlorohexane
(C) 5-Chlorohexan-2-ol
(D) 2-Chlorohexan-5-ol
Answer
C
Question. Bond angle in ethers is slightly less than
(A) Square planar angle
(B) Trigonal bipyramidal angle
(C) Tetrahedral angle
(D) None of the above
Answer
C
Question. Arrange the following compounds in increasing order of boiling point.:
Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol
(A) Propan-1-ol, butan-2-ol, butan-1-ol, pentan-1-ol
(B) Propan-1-ol, butan-1-ol, butan-2-ol, pentan-1-ol
(C) Pentan-1-ol, butan-2-ol, butan-1-ol, propan-1-ol
(D) Pentan-1-ol, butan-1-ol, butan-2-ol, propan-1-ol
Answer
A
Question. IUPAC name of the compound.

is ___________.
(A) 1-methoxy-1-methylethane
(B) 2-methoxy-2-methylethane
(C) 2-methoxypropane
(D) isopropylmethyl ether
Answer
C
Question. Which of the following species can act as the strongest base?
(A) –OH
(B) –OR
(C) –OC6H5
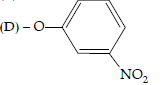
Answer
B
Question. The IUPAC name of anisole is
(A) 2-methyltoluene
(B) Methyl phenyl ether
(C) Methoxybenzene
(D) Ethoxybenzene
Answer
C
Question. Write the IUPAC name of the following compounds
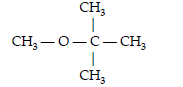
(A) 2-Methoxy-isopropane
(B) 2-Methyl-2-methoxypropane
(C) 2-Methoxy-2-methylpropane
(D) 2-Methoxy-2,2 -dimethyl ethane
Answer
C
Question. IUPAC name of m-cresol is ___________.
(A) 3-methylphenol
(B) 3-chlorophenol
(C) 3-methoxyphenol
(D) benzene-1,3-diol
Answer
A
ASSERTION AND REASON BASED MCQs
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
(A) Both A and R are true and R is the correct explanation of A
(B) Both A and R are true but R is NOT the correct explanation of A
(C) A is true but R is false
(D) A is false and R is True
Question. Assertion (A): Carboxylic acids are more acidic than phenols.
Reason (R): Phenols are ortho and para directing.
Answer
B
Question. Assertion (A): (CH3)3C−O−CH3 gives (CH3)3C−I and CH3OH on treatment with HI.
Reason (R): The reaction occurs by SN1 mechanism.
Answer
A
Question. Assertion (A): The C-O-C bond angle in ethers is slightly less than tetrahedral angle.
Reason (R): Due to the repulsive interaction between the two alkyl groups in ethers.
Answer
D
Question. Assertion (A): Ortho and para-nitrophenol can be separated by steam distillation.
Reason (R): Ortho isomer associates through intermolecular hydrogen bonding while para isomer associates through intermolecular hydrogen bonding.
Answer
C
Question. Assertion (A): Methoxy ethane reacts with HI to give ethanol and iodomethane.
Reason (R): Reaction of ether with HI follows SN2 mechanism.
Answer
A
Question. Assertion (A): Ethers behave as bases in the presence of mineral acids.
Reason (R): In ethers, oxygen consists of lone pair of electrons.
Answer
A
CASE-BASED MCQs
I. Read the passage given below and answer the following questions:
Alcohols are versatile compounds. They act both as nucleophiles and electrophiles. The bond between O-H is broken when alcohols act as nucleophiles.
(i) Alcohols as nucleophiles

(ii) The bond between C-O is broken when they act as, electrophiles. Protonated alcohols react in this manner.
Protonated alcohols as electrophiles
R-CH2-OH+H→R-CH2+OH2
Based on the cleavage of O-H and C-O bonds, the reaction of alcohols and phenols may be divided into two groups:
(a) Reactions involving cleavage of O-H bond
(b) Reactions involving cleavage of C-O bond
Acidity of alcohols and phenols
(i) Reaction with metals:Alcohols and phenols react with active metals such as sodium, potassium and aluminium to yield corresponding alkoxide/phenoxides and hydrogen.

Question. Write the IUPAC name of the following compound:
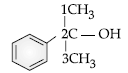
(A) 2-methyl, 2-phenyl ethanol
(B) 2-phenyl butanol
(C) 2-Phenylopropan-2-ol
(D) 1-methyl, 1-phenyl ethanol A
Answer
C
Question. Name the following reaction:
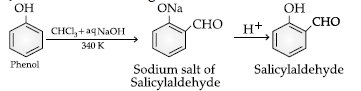
(A) Williamson’s synthesis
(B) Kolbe’s reaction
(C) Reimer-Tiemann reaction
(D) Sandmeyer’s reaction
Answer
C
Question. Given the descending order of acid strength of alcohols.
(A) RCH2OH > RR’CHOH >> RR’R”COH
(B) RCH2OH > RR’R”COH > RR’CHOH
(C) RCH2OH < RR’CHOH << RR’R”COH
(D) RCH2OH < RR’R”COH < RR’CHOH A&E
Answer
A
Question. Write down the decreasing order of reactivity of sodium metal towards primary, secondary and tertiary alcohols.
(A) 1oalc<2oalc<3oalc
(B) 1oalc>2oalc>3oalc
(C) 3oalc<1oalc<2oalc
(D) 3oalc>Ioalc < 2oalco
Answer
B
II. Read the passage given below and answer the following questions:
Oxidation of alcohols involves the formation of a carbon-oxygen double bond with cleavage of an O-H and C-H bonds.
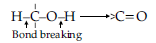
Such a cleavage and formation of bonds occur in oxidation reactions. These are also known as dehydrogenation reactions as these involve loss of dihydrogen from an alcohol molecule. Depending on the oxidising agent used, a primary alcohol is oxidised to an aldehyde which in turn is oxidised to a carboxylic acid.
Strong oxidising agents such as acidified potassium permanganate are used for getting carboxylic acids from alcohols directly. CrO3 in anhydrous medium is used as the oxidising agent for the isolation of aldehydes.
In these questions a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(C) Assertion is correct statement but reason is wrong statement.
(D) Assertion is wrong statement but reason is correct statement
Question. Assertion (A): CH3CH2OH can be converted into CH3CHO by treatment with pyridinium chlorochromate.
Reason (R): PCC is a better reagent for oxidation of primary alcohols to aldehydes.
Answer
A
Question. Assertion (A): Dehydrogenation reaction of alcohols is an oxidising reaction.
Reason (R): It involves loss of dihydrogen from alcohol.
Answer
A
Question. Assertion (A): Vapours of primary and secondary alcohols are passed through heated copper an aldehyde and ketone are formed.
Reason (R): It’s a dehydration reaction.
Answer
C
Question. Assertion (A): Tertiary alcohols do not undergo oxidation reactions.
Reason (R): They do not have the required C-H bond.
Answer
A
III. Read the passage given below and answer the following questions:
The reaction of phenol with aqueous sodium hydroxide indicates that phenols are stronger acids than alcohols and water. Due to the higher electronegativity of sp2 hybridised carbon of phenol to which –OH is attached, electron density decreases on oxygen. This increases the polarity of O–H bond and results in an increase in ionisation of phenols than that of alcohols. Now let us examine the stabilities of alkoxide and phenoxide ions. In alkoxide ion, the negative charge is localised on oxygen while in phenoxide ion, the charge is delocalised. The delocalisation of negative charge makes phenoxide ion more stable and favours the ionisation of phenol.
Question. Phenol can be distinguished from ethanol by the reaction with _____
(A) Br2/water
(B) Na
(C) Glycerol
(D) All of the above
Answer
A
Question. Which of the following is most acidic?
(A) Benzyl alcohol
(B) Cyclohexanol
(C) Phenol
(D) m-Chlorophenol
Answer
D
Question. Phenol is less acidic than_________.
(A) ethanol
(B) o-nitrophenol
(C) o-methylphenol
(D) o-methoxy phenol
Answer
B
Question. Mark the correct order of decreasing acid strength of the following compounds.
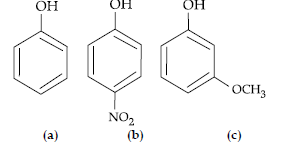
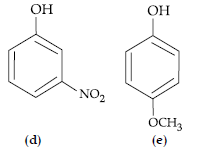
(A) e > d > b > a > c
(B) b > d > a > c > e
(C) d > e > c > b > a
(D) e > d > c > b > a
Answer
B

