Please refer to MCQ Questions Chapter 12 Thermodynamics Class 11 Physics with answers provided below. These multiple-choice questions have been developed based on the latest NCERT book for class 11 Physics issued for the current academic year. We have provided MCQ Questions for Class 11 Physics for all chapters on our website. Students should learn the objective based questions for Chapter 12 Thermodynamics in Class 11 Physics provided below to get more marks in exams.
Chapter 12 Thermodynamics MCQ Questions
Please refer to the following Chapter 12 Thermodynamics MCQ Questions Class 11 Physics with solutions for all important topics in the chapter.
MCQ Questions Answers for Chapter 12 Thermodynamics Class 11 Physics
Question. Which of the following processes is adiabatic ?
(a) Melting of ice
(b) Bursting of tyre
(c) Motion of piston of an engine with constant speed
(d) None of these
Answer
B
Question. Ice contained in a beaker starts melting when
(a) the specific heat of the system is zero
(b) internal energy of the system remains constant
(c) temperature remains constant
(d) entropy remains constant
Answer
C
Question. The gas law PV/T = constant is true for
(a) isothermal changes only
(b) adiabatic changes only
(c) both isothermal and adiabatic changes
(d) neither isothermal nor adiabatic change
Answer
C
Question. In thermodynamic processes which of the following statements is not true?
(a) In an isochoric process pressure remains constant
(b) In an isothermal process the temperature remains constant
(c) In an adiabatic process PVg = constant
(d) In an adiabatic process the system is insulated from the surroundings
Answer
A
Question. A refrigerator works between 0ºC and 27ºC. Heat is to be removed from the refrigerated space at the rate of 50 kcal/ minute, the power of the motor of the refrigerator is
(a) 0.346 kW
(b) 3.46 kW
(c) 34.6 kW
(d) 346 kW
Answer
A
Question. By what percentage should the pressure of a given mass of a gas be increased so as to decrease its volume by 10% at a constant temperature?
(a) 8.1 %
(b) 9.1 %
(c) 10.1 %
(d) 11.1 %
Answer
D
Question. The temperature at which speed of sound in air becomes double of its value at 27° C is
(a) 54°C
(b) 327°C
(c) 927°C
(d) None of these
Answer
C
Question. Two cylinders fitted with pistons contain equal amount of an ideal diatomic gas at 300 K. The piston of A is free to move, while that of B is held fixed. The same amount of heat is given to the gas in each cylinder. If the rise in temperature of the gas in A is 30 K, then the rise in temperature of gas in B is
(a) 30 K
(b) 18 K
(c) 50 K
(d) 42 K
Answer
D
Question. During an adiabatic process an object does 100J of work and its temperature decreases by 5K. During another process it does 25J of work and its temperature decreases by 5K. Its heat capacity for 2nd process is
(a) 20 J/K
(b) 24 J/K
(c) 15 J/K
(d) 100 J/K
Answer
C
Question. A thermodynamic system goes from states (i) P1, V to 2P1, V (ii) P, V1 to P, 2V1. Then work done in the two cases is
(a) zero, zero
(b) zero, PV1
(c) PV1, zero
(d) PV1, P1V1
Answer
B
Question. When 1 kg of ice at 0°C melts to water at 0°C, the resulting change in its entropy, taking latent heat of ice to be 80 cal/°C, is
(a) 273 cal/K
(b) 8 × 104 cal/K
(c) 80 cal/K
(d) 293 cal/K
Answer
D
Question. Which of the following statements about a thermodynamic process is wrong ?
(a) For an adiabatic process ΔEint = – W
(b) For a constant volume process ΔEint = + Q
(c) For a cyclic process ΔEint = 0
(d) For free expansion of a gas ΔEint > 0
Answer
A
Question. An ideal gas undergoes four different processes from the same initial state (figure). Four processes are adiabatic, isothermal, isobaric and isochoric. Out of 1, 2, 3 and 4 which one is adiabatic?
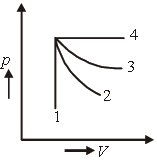
(a) 4
(b) 3
(c) 2
(d) 1
Answer
C
Question. A monoatomic gas at a pressure P, having a volume V expands isothermally to a volume 2V and then adiabatically to a volume 16V. The final pressure of the gas is :(take g = 5/3)
(a) 64P
(b) 32P
(c) P/64
(d) 16P
Answer
C
Question. Figure below shows two paths that may be taken by a gas to go from a state A to a state C.

In process AB, 400 J of heat is added to the system and in process BC, 100 J of heat is added to the system. The heat absorbed by the system in the process AC will be
(a) 500 J
(b) 460 J
(c) 300 J
(d) 380 J
Answer
B
Question. If an average person jogs, he produces 14.5 × 103 cal/min. This is removed by the evaporation of sweat. The amount of sweat evaporated per minute (assuming 1 kg requires 580 × 103 cal for evaporation) is
(a) 0.025 kg
(b) 2.25 kg
(c) 0.05 kg
(d) 0.20 kg
Answer
A
Question. In a Carnot engine efficiency is 40% at hot reservoir temperature T. For efficiency 50%, what will be the temperature of hot reservoir?

Answer
D
Question. A mass of diatomic gas (g = 1.4) at a pressure of 2 atmospheres is compressed adiabatically so that its temperature rises from 27°C to 927°C. The pressure of the gas in final state is
(a) 28 atm
(b) 68.7 atm
(c) 256 atm
(d) 8 atm
Answer
C
Question. For an isothermal expansion of a perfect gas, the value of Δp/p is equal to

Answer
B
Question. The internal energy change in a system that has absorbed 2 kcals of heat and done 500 J of work is
(a) 6400 J
(b) 5400 J
(c) 7900 J
(d) 8900 J
Answer
C
Question. A Carnot engine works first between 200°C and 0°C and then between 0°C and –200°C. The ratio of its efficiency in the two cases is
(a) 1.0
(b) 0.577
(c) 0.34
(d) 0.68
Answer
B
Question. 1 gm of water at a pressure of 1.01 × 105 Pa is converted into steam without any change of temperature. The volume of 1 g of steam is 1671 cc and the latent heat of evaporation is 540 cal. The change in internal energy due to evaporation of 1 gm of water is
(a) ≈167 cal
(b) ≈ 500 cal
(c) 540 cal
(d) 581 cal
Answer
B
Question. A gas has pressure P and volume V. It is now compressed adiabatically to 1/32 times the original volume. Given that (32)1.4 = 128, the final pressure is (g = 1.4)
(a) P/128
(b) P/32
(c) 32 P
(d) 128 P
Answer
D
Question. A perfect gas goes from a state A to another state B by absorbing 8 × 105 J of heat and doing 6.5 × 105 J of external work. It is now transferred between the same two states in another process in which it absorbs 105 J of heat. In the second process
(a) work done by gas is 105 J
(b) work done on gas is 105 J
(c) work done by gas is 0.5 × 105 J
(d) work done on the gas is 0.5 × 105 J
Answer
D
Question. Monatomic, diatomic and polyatomic ideal gases each undergo slow adiabatic expansions from the same initial volume and same initial pressure to the same final volume. The magnitude of the work done by the environment on the gas is
(a) the greatest for the polyatomic gas
(b) the greatest for the monatomic gas
(c) the greatest for the diatomic gas
(d) the question is irrelevant, there is no meaning of slow adiabatic expansion
Answer
A
Question. When heat is given to a gas in an isothermal change, the result will be
(a) external work done
(b) rise in temperature
(c) increase in internal energy
(d) external work done and also rise in temperature
Answer
A
Question. A uniform sphere is supplied heat electrically at the centre at a constant rate. In the steady state, steady temperatures are established at all radial locations r, heat flows outwards radial and is ultimatey radiated out by the outer surface isotropically. In this steady state, the temperature gradient varies with radial distance r according to
(a) r–1
(b) r–2
(c) r–3
(d) r–3/2
Answer
B
Question. At a given temperature the internal energy of a substance
(a) in liquid state is equal to that in gaseous state.
(b) in liquid state is less than that in gaseous state.
(c) in liquid state is more than that in gaseous state.
(d) is equal for the three states of matter.
Answer
B
Question. Consider p-V diagram for an ideal gas shown in figure.
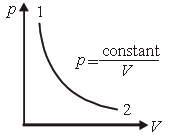
Out of the following diagrams, which figure represents the T-p diagram?

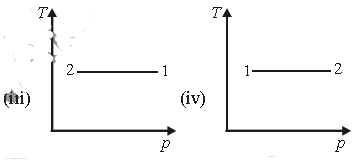
(a) (iv)
(b) (ii)
(c) (iii)
(d) (i)
Answer
C
Question. An ideal gas undergoes cyclic process ABCDA as shown in given p-V diagram. The amount of work done by the gas is
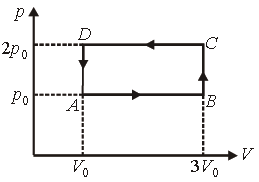
(a) 6p0V0
(b) –2p0V0
(c) +2p0V0
(d) +4p0V0
Answer
B
Question. Consider two containers A and B containing identical gases at the same pressure, volume and temperature. The gas in container A is compressed to half of its original volume isothermally while the gas in container B is compressed to half of its original value adiabatically. The ratio of final pressure of gas in B to that of gas in A is

Answer
A
Question. During an adiabatic process, the pressure of a gas is found to be proportional to the cube of its temperature. The ratio of Cp/Cv for the gas is
(a) 2
(b)5/3
(c) 3/2
(d) 4/3
Answer
C
Question. Which of the following relations does not give the equation of an adiabatic process, where terms have their usual meaning?
(a) PyT1–y = constant
(b) P1–y Ty = constant
(c) PVy = constant
(d) TVy–1 = constant
Answer
A
Question. A thermodynamic system undergoes cyclic process ABCDA as shown in fig. The work done by the system in the cycle is :
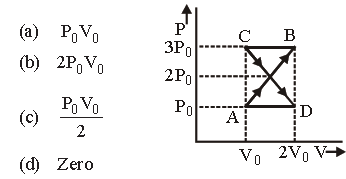
Answer
D
Question. A carnot engine having an efficiency of 1/10 as heat engine, is used as a refrigerator. If the work done on the system is 10 J, the amount of energy absorbed from the reservoir at lower temperature is
(a) 90 J
(b) 99 J
(c) 100 J
(d) 1 J
Answer
A
Question. A gas is compressed isothermally to half its initial volume. The same gas is compressed separately through an adiabatic process until its volume is again reduced to half. Then :
(a) Compressing the gas isothermally will require more work to be done.
(b) Compressing the gas through adiabatic process will require more work to be done.
(c) Compressing the gas isothermally or adiabatically will require the same amount of work.
(d) Which of the case (whether compression through isothermal or through adiabatic process) requires more work will depend upon the atomicity of the gas.
Answer
B
Question. A refrigerator works between 4°C and 30°C. It is required to remove 600 calories of heat every second in order to keep the temperature of the refrigerated space constant. The power required is: (Take 1 cal = 4.2 joules)
(a) 2.365 W
(b) 23.65 W
(c) 236.5 W
(d) 2365 W
Answer
C
Question. Thermodynamic processes are indicated in the following diagram :
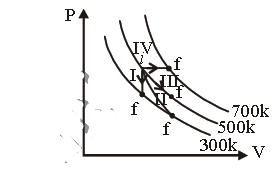
Match the following
Column-1 Column-2
P. Process I A. Adiabatic
Q. Process II B. Isobaric
R. Process III C. Isochoric
S. Process IV D. Isothermal
(a) P → C, Q → A, R → D, S → B
(b) P → C, Q → D, R → B, S → A
(c) P → D, Q → B, R → A, S → C
(d) P → A, Q → C, R → D, S → B
Answer
A
Question. The coefficient of performance of a refrigerator is 5. If the inside temperature of freezer is –20°C, then the temperature of the surroundings to which it rejects heat is
(a) 41°C
(b) 11°C
(c) 21°C
(d) 31°C
Answer
D
Question. An ideal gas is compressed to half its initial volume by means of several processes. Which of the process results in the maximum work done on the gas?
(a) Isobaric
(b) Isochoric
(c) Isothermal
(d) Adiabatic
Answer
D

We hope you liked the above provided MCQ Questions Chapter 12 Thermodynamics Class 11 Physics with solutions. If you have any questions please ask us in the comments box below.


