Please refer to Is Matter Around Us Pure Chapter 2 Class 9 Science Assignments below. We have provided important questions and answers for Is Matter Around Us Pure which is an important chapter in Class 9 Science. Students should go through the notes and also learn the solved assignment with exam solved questions provided below. All examination and class tests questions are as per the latest syllabus and books issued by CBSE, NCERT, and KVS. We have also provided Class 9 Science Assignments for all chapters on our website.
Chapter 2 Is Matter Around Us Pure Class 9 Science Assignments
Question. What is the meaning of ‘concentration of solution’?
Answer
The relative amount of solute and solvent present in a given quantity of the solution is known as the concentration of a solution.
Question. What is condenser?
Answer
It is an apparatus which converts gas into liquid by cooling it.
Question. Define dispersion medium.
Answer
It is the component which is present in excess and acts as a medium in which colloidal particles are dispersed.
Question. Define Chromatography.
Answer
The process of separation of different dissolved constituents of a mixture by absorbing them over an appropriate absorber is called, Chromatography.
Question. Name of process used to separate liquids which have difference in boiling points of less than 25°C—
Answer
Fractional distillation.
Question. Give an example of a liquid and liquid type solution.
Answer
Vinegar is a mixture of acetic acid and water.
Question. Name the types of mixtures.
Answer
Homogeneous mixture and heterogeneous mixture.
Question. What is the principle of separation?
Answer
The difference in physical or chemical properties of components of mixture is the basis of separation.
Question. Name a metal which is liquid at room temperature.
Answer
Mercury.
Question. Give chemical name of chalk and quicklime.
Answer
(i) Chalk : Calcium Carbonate
(ii) Quicklime : Calcium Oxide
Question. An element made up of only one type of—
Answer
Atom.
Question. Define the term heterogeneous.
Answer
A substance that does not have the same properties throughout the mixture is called heterogeneous.
Question. Milk of Magnesia is a-
Answer
True solution.
Question. A system which have same properties throughout is called-
Answer
Homogeneous.
Question. Hydrogen is considered as element. Why?
Answer
Hydrogen have one type of element and it cannot be broken by physical or chemical process, so it is considered as element.
Question. Classify the elements.
Answer
(1) Metals, (2) Non-metals, and (3) Metalloids.
Question. How can you test the purity of a given substance?
Answer. A pure substance always has the same taste, colour or texture at particular temperature and pressure and fixed melting or boiling point. For example : Pure water boils at 100°C but if it has some impurities then water boils at a temperature above 100°C.
Question. What is decantation? Explain.
Answer.
Decantation is the process of separating insoluble solids from liquids. A suspension of solid particles in a liquid is allowed to stand for sometime. Insoluble solid particles settle down at the bottom due to their weight. This is called sedimentation. The clear liquid is then transferred into another container, without disturbing the settled particles. In other words, clear liquid is decanted and separated from solid.
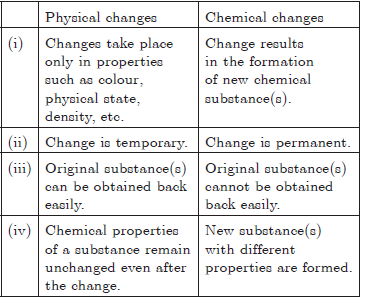
Question. Differentiate between physical and chemical changes.
or
Write any three differences between a physical change and a chemical change.
Answer. Given below is a comparison of two types of changes :
Question. Give some examples where the property : malleability and ductility of metals are used in our life.
Answer. Malleability means that metals can be hammered into sheets and foils. For example : Aluminium foils are used for wrapping food stuffs, silver foils are used for decorative purposes for sweets and fruits.Ductility means that metals can be drawn into wires. Example : Gold and silver wires are used in ornaments, aluminium and copper wires are used for conduction of electric current.
Question. Write the properties of a colloidal solution.
Answer.
(i) The size of particles is too small of a colloid; vary from 1 nm to 10 nm.
(ii) Colloid is too stable thus the particles do not settle down when left undisturbed.
(iii) Particles cannot be separated from the mixture by the process of filtration.
(iv) Colloidal solutions are translucent in nature.
(v) The particles of a .colloidal solution scatter light.
Question. Write characteristics of compounds.
Answer. Characteristics of compounds :
(i) Compounds are the substances formed by chemical combination of two or more elements.
(ii) The constituent elements are present in a fixed ratio.
(iii) A chemical reaction takes place during the formation of a compound.
(iv) Properties of a compound are different to those of its elements.
(v) Constituent elements cannot be separated by physical processes.
Question. How will you separate a mixture of common salt,camphor and iron filings. Describe the process.
Answer. Mixture of common salt, camphor and iron filings :
(i) Magnet is passed over the mixture several times.Iron filings get attached to the magnet and are separated.
(ii) Camphor is separated from the salt by sublimation.
Camphor is collected as sublimate and common salt is separated as residue.
Question. What is chromatography? What is its advantage over other methods of separation?
Answer. Chromatography is the process to separate different components of a mixture by absorbing over a suitable absorber. The main advantages of this technique is :
(i) It can be used for small amount of mixture.
(ii) Component of mixture do not get wasted.
(iii) Constituent of mixture can be identified apart from separation.
Question. 110 g solution of salt is present in 550 g of solution. Calculate the concentration of solution.
Answer. Mass of solute = 110 g
Mass of solution = 550 g

Concentration = 20% by mass
Question. Give difference between colloidal solutions and suspensions.
Answer.


Question. How much water should be mixed with 12 ml of alcohol to obtain 12% of alcohol? Calculate.
Answer.
Volume of solute = 12 ml
Concentration of solution = 12%
Volume of water = x
We know that
Concentration of solution
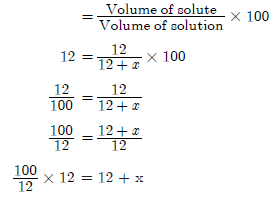
100 = 12 + x
x = 100 – 12
x = 88 ml
Volume of water = 88 ml

