Please refer to Matter in Our Surroundings Chapter 1 Class 9 Science Assignments below. We have provided important questions and answers for Matter in Our Surroundings which is an important chapter in Class 9 Science. Students should go through the notes and also learn the solved assignment with exam solved questions provided below. All examination and class tests questions are as per the latest syllabus and books issued by CBSE, NCERT, and KVS. We have also provided Class 9 Science Assignments for all chapters on our website.
Chapter 1 Matter in Our Surroundings Class 9 Science Assignments
Question. Predict the physical state of melting point of a substance is below the room temperature.
Answer
Ice.
Question. We can get the smell of perfume sitting several metres away, why?
Answer
This is because perfumes diffuse very fast and can reach to people sitting several metres away.
Question. The boiling point of alcohol is 78°C. What is this temperature on Kelvin scale?
Answer
K = °C + 273 = 78 + 273 = 351 K
Question. We can easily move our hand in the air but to do the same through a solid block of wood. We need a karate expert. Why?
Answer
In air, the inter-particle attractive forces are negligible and hence, it is easy to separate the particles in air and we can easily move our hand in air. The interparticle forces are very strong in solids. So, it is not easy to separate the particles and it is not easy to move our hand through a solid block of wood.
Question. Give two examples of diffusion.
Answer
Milk drops dissolved in water and perfume sprayed in a room.
Question. Express the boiling point of water in Celsius as well as Kelvin scale.
Answer
100°C and 373 K.
Question. Name the state of water at 100 degree Celsius, zero degree Celsius and 4 degree Celsius.
Answer
The state of water at 100 degree Celsius is gas, at 0 degree Celsius it is solid and at 4 degree Celsius it is liquid.
Question. Name two processes from which it may be concluded that the particles of a gas move continuously.
Answer
Compressibility and Brownian movement.
Question. Is it possible to turn a liquid into vapour without heating?
Answer
Yes, by the process of evaporation as evaporization of water occur below the boiling point under atmospheric pressure.
Question. What is diffusion?
Answer
The intermingling of molecules of one substance with that of the other is called diffusion.
Question. What happens to the rate of diffusion if the temperature is increased?
Answer
With increased temperature, the rate of diffusion also increases as the particles gain energy and vibrate more.
Question. What is dry ice?
Answer
Solid carbon dioxide obtained by cooling and applying pressure on carbon dioxide gas. It does not melt so it is called dry ice.
Question. What is humidity?
Answer
The air holds water vapour, this air with water is called humid air and the amount of water vapour present in the air is called humidity.
Question. What is normal atmospheric pressure?
Answer
The atmospheric pressure at sea level is 1 atmosphere and taken as the normal atmospheric pressure.
Question. Write the SI unit of temperature?
Answer
Kelvin.
Question. Give the temperature at which water exists in two different phases/states.
Answer
(i) At 0°C water can be in solid or in liquid state.
(ii) At 100°C water can be in liquid or in gaseous state.
Question. What are fluids?
Answer
The states of matter that can flow due to less intermolecular force of attraction are liquids and gases and are called fluids.
Question. Define matter.
Answer
Anything that occupies space and has mass and is felt by senses is called matter.
Question. Why are light and sound not considered as matter?
Answer
Light and sound are not considered as matter because they have no mass and do not occupy space.
Question. What happens if you put copper sulphate crystals in water?
Answer
Copper sulphate crystals mixed between the spaces of molecules of water and disappear.
Question. Give state of a matter if this substance has neither a fixed shape nor a fixed volume.
Answer
Gas.
Question. What do you mean by vapour?
Answer
A substance that is found in gaseous state only at room temperature is called vapour.
Question. Is it true to say that fluorescent tube contains plasma? Explain
Answer. It is right to say that fluorescent tube contains plasma. As fluorescent tube has helium or some other rare gas.The particles of the gas get ionized in the presence of high voltage applied. These charged particles are called plasma which glows.
Question. A karate expert can easily move his hand through a solid block of wood but we cannot. Why?
Answer. In a solid block of wood, the inter-particle forces are very strong and hence, it is not easy to separate the particles. Therefore, it is not easy to move our hand through a solid block of wood, only a karate expert can do it as he has expertise in this.
Question. What is latent heat of fusion?
Answer. The heat required to change 1 kg of a solid substance into liquid state at the melting point of the substance.
For example : Amount of heat required to melt ice at 0°C into water, at 0°C will be known as the latent heat of fusion of ice.
Question. What is compressibility? How it is negligible in solids?
Answer. Compressibility is the ability of a substance to be reduced to its volume under pressure. Solids are incompressible as their particles are held together. So,
we can tell that compressibility is negligible in solids.
Question. Two cubes of ice are pressed hard between two palms and after releasing the pressure, the cubes join together. Why?
Answer. Pressure is directly proportional to temperature when we apply pressure, temperature increases then the ice in contact melts and it turns into water. When pressure is removed, the temperature decreases again and melted ice again freezes. Hence, cubes join together.
Question. What is the reason that “Ice has lower density than water”?
Answer.The mass per unit volume of a substance is called density (density = mass/volume). The density of substance decreases as the volume of a substance increases. Space between particles increases when water changes into ice. These spaces are larger as compared to the spaces present between the particles of water. Thus, the volume of ice become greater as compared to the water. Hence, the density of ice become lower than that of water. And, a substance with lower density than water can float on water.
Thus, ice floats on water.
Question. Why does the temperature remain constant during the change of state, for any substance?
Answer.On increasing the temperature of solids, the kinetic energy of the particles increases which is used up in changing the state as it overcome the forces of attraction between the particles, therefore, the temperature remains constant during the change of state.
Question. Why cannot you smell its perfume at a short distance when incense stick is not lighted?
Answer. The particles of the perfume (matter) do not have sufficient energy to drift through the air. Thus, we cannot smell it at a few steps away from incense stick.
Question. How can you show that evaporation causes cooling?
Answer. When we put some acetone on our hand, after some time we will feel coolness on our hand because the acetone absorbs kinetic energy from our hand and evaporates and evaporation causes cooling.
Question. What do you mean by latent heat of vaporization?
Answer. The latent heat of vaporization of a liquid is the quantity of heat in joules required to convert 1 kilogram of the liquid to vapour or gas at its boiling point, without any change in temperature.
Question. Explain, why solids have fixed shape but liquids and gases do not have fixed shape?
Answer. Solids have fixed shape due to strong intermolecular force of attraction between them. The liquids and gases have molecules with less intermolecular force of attraction, and hence they can flow and take shape of the container.
Question. Liquids and gases can be compressed but it is difficult to compress solids. Why?
Answer. Liquids and gases have intermolecular space; on applying pressure externally on them the molecules can come closer thereby minimizing the space between them. But in case of solids, there is no intermolecular space to do so.
Question. Is it not proper to regard the gaseous state of ammonia as vapours? Explain.
Answer. The gaseous state of a substance can be regarded as vapours only in case it is a liquid at room temperature. Since ammonia is a gas at room temperature, its gaseous state cannot be regarded as vapours. Naphthalene is volatile solid and has a tendency to sublime. So, it changes into vapours completely, thus disappear into the air and no solid is left.
Question. Cotton is solid but it floats on water. Why?
Answer. Cotton has large number of pores where air is trapped.This process reduces cotton’s density and increase the volume. Therefore, cotton floats on water. But, when these pores get filled with water, it starts sinking.
Question. Why do we see water droplets on the outer surface of a glass containing ice-cold water?
Answer. If we take some ice-cold water in a glass, after some time we will see small droplets of water deposited on the outer walls of the glass. Because water vapour present in air come into the contact of cold wall of glass, lose energy and converted into liquid state which can be seen in the form of small droplets.
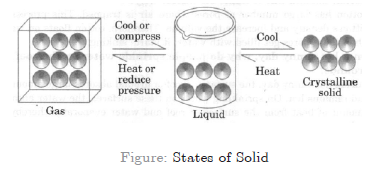
Question. Pressure and temperature determine the state of a substance. Explain this in detail.
Answer. Any matter, i.e. solid, liquid or gas when experiences an increase in temperature then they change their state.
Example :
When we take ice cubes in a beaker or heat them slowly, the temperature increases and ice melts to form liquid. We heat this liquid further it will become steam.
On lowering down the temperature of any matter,show change in their state.
Example :
We take the steam that is coming out of boiling water and allow it to cool down, it condenses to form water and on further cooling of this water we get ice. On applying pressure and reducing temperature we can liquefy gases or change them into solid.
Example : We take carbon dioxide gas, reduce its temperature and apply lot of pressure on it so that it changes into solid carbon dioxide, called dry ice, which is used as refrigerant for cooling.
If pressure on it is decreased it directly changes into gas.
In LPG cylinders, the petroleum gas is cooled and with lot of pressure changes it into liquid state.
While using this LPG, we release the pressure exerted on it and hence, it comes out in the form of gas.
Question. Give difference between Evaporation and Boiling.
Answer.
Evaporation Boiling
1. It takes place at any It takes place at definite temperature called boiling point of liquid.
place.
2. Temperature of liquid Temperature of liquid does not change during boiling.
decreases during
evaporation.
3. Evaporation is a Boiling is the bulk phenomenon; it takes place in the whole mass of the liquid.
surface phenomenon;
it takes place only
at the surface of the
liquid.
4. Evaporation is a slow Boiling is a rapid and violent process.
and silent process.
Question. Explain the inter-conversion of three states in terms of force of attraction and kinetic energy of the molecules.
Answer. The force working between the particles of a matter is called intermolecular force. Intermolecular forces are strong in solids and the particles are close to each other and thus make the substance rigid. In liquids,intermolecular force is less than solids and more than gases. So, they cannot have rigid shape and kinetic energy of the molecules is not enough to hold gas in open container.