Please refer to Atoms And Molecules Chapter 3 Class 9 Science Assignments below. We have provided important questions and answers for Atoms And Molecules which is an important chapter in Class 9 Science. Students should go through the notes and also learn the solved assignment with solved questions provided below. All examination and class tests questions are as per the latest syllabus and books issued by CBSE, NCERT, and KVS. We have also provided Class 9 Science Assignments for all chapters on our website.
Chapter 3 Atoms And Molecules Class 9 Science Assignments
Question. How did the scientist lay the foundation of chemical sciences? Name the scientist.
Answer
Antoine Laurent Lavoisier laid the foundation of chemical science by establishing two important laws of chemical combination.
Question. Give the full form of amu?
Answer
The full from of amu is atomic mass unit.
Question. Define law of conservation of mass.
Answer
Law of conservation of mass states that, ‘Mass is neither created nor destroyed in a chemical reaction.’
Question. Why is it not possible to see an atom with naked eyes?
Answer
Because an atom is too small, i.e., the atomic radii of an atom is of the order 10–19 m to 10–9 m.
Question. Define law of constant proportion.
Answer
Law of constant proportion states that, ‘In a pure chemical substance, the elements are always present in definite proportions by mass.’
Question. What do we get if 3 atoms of oxygen unite into a molecule, instead of usual 2 ?
Answer
We get (O3) ozone.
Question. Name two atoms which exist as independent atoms.
Answer
Noble gases such as argon (Ar) and helium (He) exist as independent atoms.
Question.. What is the number of electrons in Mg atom and Mg2+ ion?
Answer
Mg = 12e–
Mg2+ = 10e–
Question. Write atomicity of the following :
(i) Sulphur, (ii) Phosphorus
Answer
(i) Polyatomic, (ii) Tetra atomic.
Question. Define atomicity.
Answer
The number of atoms present in one molecule of an element or a compound is known as its atomicity.
Question. What are polyatomic ions? Give two examples.
Answer
A group of atoms having a charge is known as polyatomic ion.
Examples : (NH4)+ (SO4)2–
Question. Give one relevant reason, why scientists choose 1/16th of the mass of an atom of naturally occurring oxygen as the atomic mass unit?
Answer
Initially, 1/16thof the mass of naturally occurring oxygen was taken as the atomic mass unit because this unit gave masses of most of the elements as whole numbers.
Question. State the law of conservation of mass.
Answer
This law states that the mass can neither be created nor destroyed in a chemical reaction. That is ‘bass of reactants is always equal to mass of products.
Question. State the number of hydrogen atoms in 1 g of hydrogen.
Answer
One gram of hydrogen = One mole
= 6.022 × 1023 atoms
Question. What is molar mass? What are its units?
Answer
The mass of one mole of a substance is called its molar mass. Its unit is gram per mole (gmol–1).
Question. The relative atomic mass of oxygen atom is 16.Explain its meaning.
Answer
The relative atomic mass of an atom is the average masses of the atom, as compared to 1/12th the mass of one carbon-12 atom.
Question. Distinguish between molecular mass and molar mass.
Answer
The molecular mass of a substance is the sum of the atomic masses of all atoms in a molecule, whereas the mass of 1 mole of any substance is called its molar mass.
Question. Define the atomic mass unit.
Answer
The mass of 1/12th part of C-12 is equivalent to one atomic mass unit. Previous, it was denoted by symbol ‘amu’ but nowadays it is denoted by symbol ‘u’.
Question. What is the building block of all matter?
Answer
Atom is the building block of all matter.
Question. What is the measuring unit of atomic radius?
Answer
Nanometre (nm) is the measuring unit of atomic radius.
Question. If 9 g of water is decomposed, how many grams of hydrogen and oxygen are obtained?
Answer
If 9 g of water is decomposed, 1 g of hydrogen and 8 g of oxygen are always obtained.
Question. How was the relative atomic mass determined?
Answer
Relative atomic masses were determined by using the laws of chemical combinations and the compound formed.
Question. What did Antoine L. Lavoisier observe regarding the formation of compound?
Answer
He noted that many compounds were composed of two or more elements. Each compound had the same
elements in the same proportions.
Question. Name the gas which gives the lightest positively charged particle.
Answer
Hydrogen.
Question. Hydrogen and oxygen combine in the ratio of 1 : 8 by mass to form water. What mass of oxygen gas would be required to react completely with 3 g of hydrogen gas?
Answer
1 g of hydrogen reacts with oxygen = 8 g
3 g of hydrogen reacts with oxygen = 8 × 3 g= 24 g
Question. Write the chemical symbols of two elements which are formed from the first letter of the elements’ name.
Answer
N (Nitrogen), F (Fluorine), I (Iodine), O (Oxygen) (any two).
Question. Why atoms form ions?
Answer
Atoms get stability by acquiring the stable electronic configuration of the nearest noble gas for which either they lose electrons or gain electrons and thus acquire noble gas configuration.
Question. What is the full form of IUPAC?
Answer
International Union of Pure and Applied Chemistry.
Question. What is the significance of symbol of an element? Explain with an example.
Answer.
(i) The symbol of an element represents the name of the element.
(ii) It represents one atom of the element.
(iii) It represents a definite mass of the element.
For example :
(i) The symbol ‘H’ represents the element hydrogen.
(ii) The symbol ‘H’ represents one atom of the element hydrogen.
(iii) The symbol ‘H’ represents 1u.
Question. What is the ratio by mass of combining elements in H2O, CO2 and NH3 ?
Answer. H2O ratio by mass of combining elements
= 2 : 16 = 1 : 8 (H : O)
CO2 ratio by mass of combining elements
= 12 : 32 = 3 : 8 (C : O)
NH3 ratio by mass of combining elements
= 14 : 3 = 14 : 3 (N : H)
Question. How can Dalton’s atomic theory explain the Law of Constant Proportions?
Answer. According to Dalton’s atomic theory, atoms of the same elements are same. Also atoms combine in whole number. This means that the atoms can combine with each other in a simple fixed ratio to form molecules.
Question. Calculate the following in 5.6 g of nitrogen :
(a) Number of moles of nitrogen
(b) Number of molecules of nitrogen
(c) Number of atoms of nitrogen
Answer.
(a) Molar mass of nitrogen = 14 g
5.6 g of nitrogen =5.6/14 = 0.4 mole
(b) 28 g of nitrogen = 6.022 × 1023 molecules
5.6 g of nitrogen = 2.15 × 1022 molecules
(c) 14 g of nitrogen = 6.023 × 1023 atoms
5.6 g of nitrogen = 4.30 × 1023 atoms
Question. What is meant by a molecule? Give examples.
Answer. A molecule is the smallest particle of an element or a compound capable of independent existence under ordinary conditions. It shows all the properties of the substance, e.g., molecule of oxygen is O2, ozone is O3, phosphorus is P4, sulphur is S8, etc.
Question. Find the molecular mass of the following :
H2, O2, CH4, CH3OH, CO2, HCl, Na2O, MgCl2, NaF, Na2CO3, NaNO3, H2SO4
Answer.


Question. Define one mole, give its relationship with Avogadro constant.
Answer. One mole of any species (atoms, molecules, ions or particles) is that quantity in number having a mass equal to its atomic or molecular mass in grams. The number of particles (atoms, molecules or ions) present in 1 mole of any substance is fixed, with a value of 6.022 × 1023. This number is called Avogadro constant
Question. What is the similarity between chlorine molecule,nitrogen molecule and hydrogen molecule?
Answer. Chlorine molecule, nitrogen molecule and hydrogen molecule are diatomic molecules. These are formed by the union of two atoms of the same element.
Question. What is the mass of :
(a) 0.2 mole of oxygen atoms?
(b) 0.5 mole of water molecules?
Answer.
(a) 1 mole of oxygen atoms = 1 × 16 = 16 g
0.2 mole of oxygen atoms = 16 g × 0.2 = 3.2 g
(b) 1 mole of water (H2O) molecules
= 2 × 1 g + 1 × 16 g = 18 g
0.5 mole of water (H2O) molecules
= 18 g × 0.5 = 9.0 g
Question. Write the names of the following compounds :
(a) NiS
(b) Mg(NO3)2
(c) Na2SO4
(d) Al(NO3)3
(e) K3PO4
(f) Ca3N2
Answer.
(a) Nickel sulphide,
(b) Magnesium nitrate,
(c) Sodium sulphate,
(d) Aluminium nitrate,
(e) Potassium phosphate,
(f) Calcium nitride.
Question. The atomic mass of an element is in fraction.” What does it mean?
Answer. If the atomic mass of an element is in fraction, this means that it exists in the form of isotopes. The atomic mass of such element is the average of atomic masses of its isotopes and is generally in fraction.
Question. Define formula unit mass. Calculate formula unit mass of NaCl (atomic mass of Na = 23u, Cl = 35.5u).
Answer.The formula unit mass is same as molecular mass which is equal to the sum of masses of atoms present in a formula unit. Formula unit mass of NaCl = (23 + 35.5) = 58.5u.
Question. Calculate the mass of the following :
(i) 2 moles of carbon dioxide.
(ii) 6.022 × 1023 molecules of carbon dioxide.
Answer.
(i) Molar mass of CO2 = 12 + 2 × 16 g = 44 g
1 mole of carbon dioxide = 44 g
2 mole of carbon dioxide = 44 g × 2 = 88 g
(ii) Molar mass of CO2 = 44 g
= 6.022×1023 molecules of carbon dioxide
Question. All elements have charged valency. Explain.
Answer. No, all elements do not form ions thus they, do not have a charge. For example : Carbon has a valency of 4 and nitrogen has a valency of 3. Non-metals are formed without a charged valency. Example : In carbon tetrachloride, carbon has valency of 4 and chlorine has a valency of 1.
Question. Write the symbols of the following elements :
Aluminium, Argon, Barium, Bromine, Beryllium,Calcium, Cobalt, Chlorine, Chromium, Helium,Lithium, Magnesium, Manganese, Neon, Nickel,Silicon, and Platinum.
Answer.
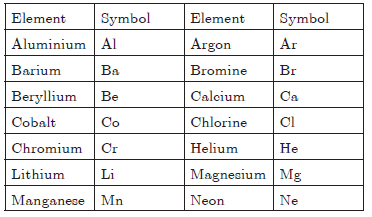
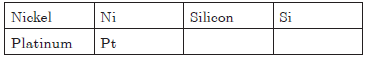
Question. Give two drawbacks of Dalton’s atomic theory.
Answer. Drawbacks of Dalton’s atomic theory :
(i) According to modern theory, atom is not the ultimate indivisible particle of matter. Atoms are divisible, i.e., they are themselves made-up of particles (protons, electrons, neutrons, etc.).
(ii) The assumption that the atoms of the same element have same mass does not hold good, in case of isotopes of an element.
Question. Calculate molar mass of sulphuric acid.
Answer. Formula of sulphuric acid = H2SO4
Molar mass of H2SO4 = 2 × mass of H
+ 1 × mass of S + 4 × mass of O
= 2 × 1 + 1 × 32 + 4 × 16
= 2 + 32 + 64 = 98 gmol–1
Question. Give the electronic configuration of : Al atom and its ion.
Answer.Al atom and its ion
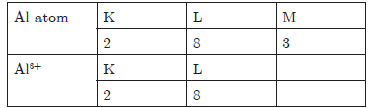
Question. Write three points of difference between an atom and a molecule.
or
What is the difference between an atom and a molecule?
Answer.
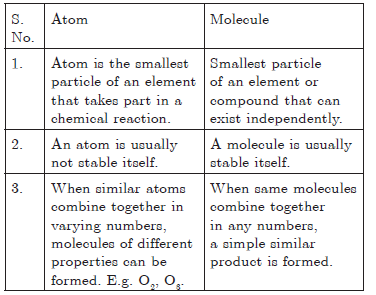
Question. What are molecules? Give brief explanation of the arrangement of the constituent atoms in the molecules.
Answer. A molecule is the smallest particle of an element or compound which is stable in normal conditions. And it can freely show all the properties of that element or compound. It may be made up of one, two or more atoms. Molecule with one atom called monoatomic.
E.g. helium, neon, etc.
Molecule with two atoms called diatomic. E.g.
Cl2, O2. Similarly, there are molecules containing three atoms (CO2), four atoms (P4) and so on.
Question. The mass of one molecule of a substance is 4.65 × 1023 grams. What is its molecular mass?
Answer. Mass of 1 molecule of a substance = 4.65 × 1023 grams
Mass of 6.023 × 10–23 molecules of a substance
= 4.65 × 1023 × 6.023 × 10–23
= 28 g
Molecular mass of the substance = 28 g
Question. Chlorine occurs in nature in two isotopic forms with masses 35u and 37u in the ratio of 3 : 1. What should be the mass of chlorine atom?
Answer.

Question. An element 12X24 loses two electron to form a cation which combines with the anion of element 17Y35 formed by gaining an electron.
(a) Write the electronic configuration of element X.
(b) Write the electronic configuration of the anion of element Y.
(c) Write the formula for the compound formed by combination of X and Y.
Answer.
(a) X = 2, 8, 2
(b) Y– = 2, 8, 8
(c) XY2
Question. Calculate the formula unit masses of ZnO, Na2O, K2CO3 given atomic masses of Zn = 65u, Na = 23u, K = 39u, C = 12u, and 0 = 16u.
Answer.
Formula unit mass of ZnO = 1 × 65u + 1 × 16u = 81u
Formula unit mass of Na2O = 2 × 23u + 1 × 16u = 62u
Formula unit mass of K2CO3
= 2 × 39u + 1 × 12u + 3 × 16u
= 138u
Question. If one mole of carbon atoms weighs 12 grams, what is the mass (in gram) of 1 atom of carbon?
Answer. 1 mole of carbon weighs = 12 g
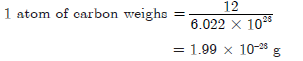
Question. What is the mass of :
(a) 1 mole of nitrogen atoms?
(b) 4 moles of aluminium atoms (atomic mass of aluminium = 27)?
(c) 10 moles of sodium sulphite (Na2SO3)?
Answer.
(a) 1 mole of nitrogen atoms
= 1 × gram atomic mass of nitrogen atom
= 1 × 14 g = 14 g
(b) 4 moles of aluminium atoms
= 4 × gram atomic mass of aluminium atoms
= 4 × 27 g = 108 g
(c) 10 moles of sodium sulphite (Na2SO3)
= 10 (2 × gram atomic mass of Na
+ 1 × gram atomic mass of sulphur
+ 3 × gram atomic mass of oxygen)
= 10 (2 × 23 g + 1 × 32 g + 3 × 16g)
= 10 (46 g + 32 g + 48 g)
= 10 × 126 g = 1260 g
Question. Give the postulates of Dalton’s atomic theory.
Answer. Every element is composed of extremely small particles called atoms. Atoms of a given element are identical,both in mass and properties. Different chemical elements have different kinds of atoms; in particular, their atoms have different masses.Atoms cannot be created, destroyed or transformed into atoms of other elements. Compounds are formed when atoms of different elements combine with each other in small whole number ratios. The relative
number and kinds of atoms in a given compound are constant.
Question. (a) Give one point of difference between an atom and an ion.
(b) Give one example each of a polyatomic cation and an anion.
(c) Identify the correct chemical name of FeSO3: Ferrous sulphate, Ferrous sulphide, Ferrous sulphite.
(d) Write the chemical formula for the chloride of magnesium.
Answer.
(a) An atom is electrically neutral while an ion is electrically charged particle.
(b) (i) Polyatomic cation : (NH4)+
(ii) Polyatomic anion : (SO4)2–
(c) Ferrous sulphite
(d) MgCl2 (Magnesium chloride)
Question. When 3.0 g of magnesium is burnt in 2.00 g of oxygen, 5.00 g of magnesium oxide is produced. What mass of magnesium oxide will be formed when 3.00 g
magnesium is burnt in 5.00 g of oxygen? Which law of chemical combination will govern your answer? State the law.
Answer.
When 3.0 g of magnesium is burnt in 2.00 g of oxygen, 5.00 g of magnesium oxide is produced. It means magnesium and oxygen are combined in the ratio of 3 : 2 to form magnesium oxide.
Thus, when 3.00 g of magnesium is burnt in 5.00 g of oxygen, 5.00 g of magnesium oxide will be formed and the remaining oxygen will be left unused. It is governed by law of definite proportions.
It states that in a chemical substance, the elements are always present in definite proportions by mass.
Question. A sample of ethane (C2H6) gas has the same mass as 1.5 × 1020 molecules of methane (CH4). How many (C2H6) molecules does the sample of gas contain?
Answer.
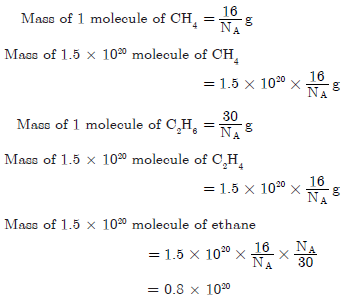
Question. (a) Calculate the number of molecules of SO2 present in 44 g of it.
(b) If one mole of oxygen atoms weighs 16 grams, find the mass of one atom of oxygen in grams.
Answer.
(a) Molecular mass of SO2 = Atomic mass of S + 2 × Atomic mass of O
= 32 + 2 × 16 = 64u
Molar mass = 64 g
Number of molecules, N
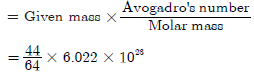
= 4.14 × 1023 molecules
(b) One mole of oxygen contains 6.022 × 1023 atoms of oxygen
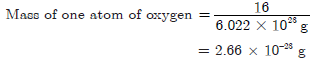
Question. Sodium is represented as 23Na11.
(a) What is its atomic mass?
(b) Write its gram atomic mass.
(c) How many atoms of Na will be there in 11.5 g of the sample?
Answer.
(a) Atomic mass = 23u
(b) Gram atomic mass = 23 g
(c) Given mass = 11.5 g
Molar mass = 23 g
Number of atoms (N)
= 3.011 × 1023 atoms


