Please refer to the States of Matter Revision Notes given below. These revision notes have been designed as per the latest NCERT, CBSE and KVS books issued for the current academic year. Students will be able to understand the entire chapter in your class 11th Chemistry book. We have provided chapter wise Notes for Class 11 Chemistry as per the latest examination pattern.
Revision Notes Chapter 5 States of Matter
Students of Class 11 Chemistry will be able to revise the entire chapter and also learn all important concepts based on the topic wise notes given below. Our best teachers for Grade 11 have prepared these to help you get better marks in upcoming examinations. These revision notes cover all important topics given in this chapter.
Change in state : It is over all effect of Intermolecular forces, molecular Interactional energy & thermal energy
Measurable properties of gases : P,V, T, n, Viscosity, specific heat are some measurable properties.
Gas Laws : The quantitative relationship b/w any two of the variables (V, n, P,T) when other two are constant.
Boyle’s Law : The pressure of fixed msss of gas varies inversely with the volume at constant T. P α 1/V(n,T const.) P1V1 = P2V2
Charle’s Law : At constant P, the volume of fixed amount of gas varies directly with its absolute temperature.
V α T or V / T = cons tan , V1 /T1 = V2 /T2
Gay lussac’s Law : At constant V, The pressure of fixed amount of gas varies directly with its absolute temperature.
P α T or P / T = const, P1 /T1 = P2 /T2
Ideal gas equation : The relationship b/w P, V and T by Gas Laws PV= nRT.
Avogadro’s Law : At given T and P, the volume of gas varies directly to the amount of gas . V α n ( P, T constant)
Dalton’s Law of partial persure : The pressure enerted by a mixture of non reacting gases is equal to the sum of their partial pressure at constant (V,T)
P (total ) = P1 + P2 + P3 + ………. (T, V, constant)
Kinetic Molecular theory :
• Gases consist of large number of identical particles (atoms or molecules) that are so small that the actual volume of the molecules is negligible in comparison to the empty space between them.
• There is no force of attraction between the particles of a gas at ordinary temperature and pressure
• Particles of a gas are always in constant and random motion
• Pressure is exerted by the gas as a result of collision of the particles with the walls of the container
• Collisions of gas molecules are perfectly elastic
• At any particular time, different particles in the as have different speeds and hence different kinetic energies
• Average kinetic energy of the gas molecules is directly proportional to the absolute temperature
Real Gases : The gases which deviates from Ideal behavior at higher pressure and low temperature b/c of force of attraction b/w molecules increases .
Compressibility factor (Z) : It determine extent of devation of real gases from Ideal gas behavior : Z = PV / nRT for ideal gas Z=1, for Nonideal gas Z< 1, Z > 1
Vander waal’s Equation :
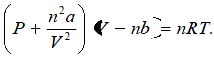
Critical Temperature : (Tc) The temperature above which a gas cannot be liquefied whatever high pressure may be
Critical Pressure : The minimum pressure required to liquity a gas at its critical temperature.
Critical Volume : The volume of 1 mole of gat at Tc, Pc.
Super cooled liquids : The liquids which are cooled to a temperature below its freezing point without freezing .
Elastic Collision : The collisions in which no loss of K.E. only there is transfer of energy.
Vapour pressure : The equilibrium pressure by vapour of liquid in a container at given temperature (T)
At higher altitude : The b.p. of water decreases b/c the atmospheric pressure is less than one atmosphere.
Surface Tension (V) : It is force acting per unit length perpendicular to the line drawn on the surface : (Nm-1) : It decreases with increases in T, it increases with increase in external pressure, b/c of it falling drops of liquid are spherical, liquid in capillary tube rises.
Viscosity (η) : It is resistance offered to the flow of liquid due to friction b/w layer of fluids . F= n.A.dv / dn
Effect of T & P on viscosity : It decreases with increase in T, and increases with increase in P.
Low M.P. & B.P. of molecular liquids is due to low magnitude of molecular interaction energy.
Important Points
1. Intermolecular forces : Intermolecular forces are the forces of attraction and repulsion between interacting particles (atoms and molecules). This term does not include the electrostatic forces that exist between the two ppositely charged ions and the forces that hold atoms of a molecule together i.e., covalent bonds. Attractive intermolecular forces are known as van der Waals forces, in honour of Dutch scientist Johannes van der Waals (1837-1923).
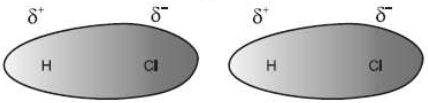
2. Dipole – Dipole Forces: Dipole-dipole forces act between the molecules possessing permanent dipole. Ends of the dipoles possess ―partial charges‖ and these charges are shown by Greek letter delta (δ)
3. Dipole -Induced Dipole Forces: This type of attractive forces operate between the polar molecules having permanent dipole and the molecules lacking permanent dipole. Permanent dipole of the polar molecule induces dipole on the electrically neutral molecule by deforming its electronic cloud.Thus an induced dipole is developed in the other molecule

4. Dispersion Forces or London Forces: Atoms and nonpolar molecules are electrically symmetrical and have no dipole moment because their electronic charge cloud is symmetrically distributed. But a dipole may develop momentarily even in such atoms and molecules. This can be understood as follows. Suppose we have two atoms ‘A‘ and ‘B‘ in the close vicinity of each other . It may so happen that momentarily electronic charge distribution in one of the atoms, say ‘A‘, becomes unsymmetrical i.e., the charge cloud is more on one side than the other This results in the development of instantaneous dipole on the atom ‘A‘ for a very short time. This instantaneous or transient dipole distorts the electron density of the other atom ‘B‘, which is close to it and as a consequence a dipole is induced in the atom ‘B‘. The temporary dipoles of atom ‘A‘ and ‘B‘ attract each other. Similarly temporary dipoles are induced in molecules also. This force of attraction was first proposed by the German physicist Fritz London, and for this reason force of attraction between two temporary dipoles is known as London force. Another name for this force is dispersion force.
5. Hydrogen bond: Hydrogen bond is represented by a dotted line (– – –) while a solid line represents the covalent bond. Thus, hydrogen bond can be defined as the attractive force which binds hydrogen atom of one molecule with the electronegative atom (F, O or N) of another molecule this is special case of dipole-dipole interaction.
6. Boyleís Law (Pressure – Volume Relationship): At constant temperature, the pressure of a fixed amount (i.e., number of moles n) of gas varies inversely with its volume. This is known as Boyleís law.
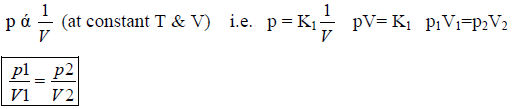
7. Charles Law (Temperature – Volume Relationship): It states that pressure remaining constant, the volume of a fixed mass of a gas is directly proportional to its absolute temperature.i.e. V ά T (at constant P & V)

8. Gay Lussacís Law (Pressure-Temperature Relationship): It states that at constant volume, pressure of a fixed amount of a gas varies directly with the temperature. Mathematically, . P ά T (at constant V & n)
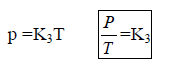
9. Avogadro Law (Volume – Amount Relationship): It states that equal volumes of all gases under the same conditions of temperature and pressure contain equal number of molecules. i.e. V ά n i.e.V = k4n
Since volume of a gas is directly proportional to the number of moles; one mole of each gas at standard temperature and pressure (STP)* will have same volume. Standard temperature and pressure means 273.15 K (0oC) temperature and 1 bar (i.e., exactly 105 pascal) pressure
10. Ideal gas: A gas that follows Boyle’s , Charles law and Avogadro law strictly.
11. Ideal Gas Equation: pV = n RT
12. Universal Gas Constant : R is called gas constant. It is same for all gases. R= 8.314 Pa m3 K–1 mol–1 = 8.314 × 10–2 bar L K–1 mol–1 = 8.314 J K–1 mol–1
13. Equation of state:
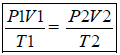
14. Density and Molar Mass of a Gaseous Substance: M= dRT / P
15. Daltonís Law of Partial Pressures: It states that the total pressure exerted by the mixture of non-reactive gases is equal to the sum of the partial pressures of individual gases i.e., the pressures which these gases would exert if they were enclosed separately in the same volume and under the same conditions of temperature. In a mixture of gases, the pressure exerted by the individual gas is called partial pressure. Mathematically, pTotal = p1+p2+p3+……(at constant T, V) where pTotal is the total pressure exerted by the mixture of gases and p1, p2 , p3 etc. are partial pressures of gases.
16. KINETIC MOLECULAR THEORY OFGASES:
• Gases consist of large number of identical particles (atoms or molecules) that are so small and so far apart on the average that the actual volume of the molecules is negligible in comparison to the empty
space between them. They are considered as point masses. This assumption explains the great compressibility of gases.explains the great compressibility of gases.
• There is no force of attraction between the particles of a gas at ordinary temperature and pressure. The support for this assumption comes from the fact that gases expand and occupy all the space available to them.
• Particles of a gas are always in constant and random motion. If the particles were at rest and occupied fixed positions, then a gas would have had a fixed shape which is not observed.
• Particles of a gas move in all possible directions in straight lines. During their random motion, they collide with each other and with the walls of the container. Pressure is exerted by the gas as a result of collision of the particles with the walls of the container.
• Collisions of gas molecules are perfectly elastic. This means that total energy of molecules before and after the collision remains same
17. Behaviour Of Real Gases: Deviation From Ideal Gas:Real gases show deviations from ideal gas law
(a)Pressure correction: pressure exerted by the gas is lower than the pressure exerted by the ideal gas.

(b)Volume Correction: (V–nb) where nb is approximately the total volume occupied by the molecules themselves. Here, b is a constant.
18. Van der Waals equation.equation :
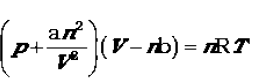
Constants a and b are called van der Waals constants
19. Significance of Vander wall parameter: Vander wall parameter a is the measure of intermolecular forces while b is the measure of effective size of gaseous
molecules Unit of a = bar L3 mol-2 Unit of b = L mol-1
20. The deviation from ideal behaviour can be measured in terms of compressibility factor Z, which is the ratio of product pV and nRT.
Mathematically Z= pV / nRT
21. Boyle temperature : The temperature at which a real gas obeys ideal gas law over an appreciable range of pressure is called Boyle temperature or Boyle point.
22. Critical temperature (Tc)of a gas is highest temperature at which liquifaction of the gas first occurs. Liquifaction of so called permanent gase Volume of one mole of the gas at critical temperature is called critical volume (Vc) and pressure at this temperature is called critical pressure (pc). The critical temperature, pressure and volume are called critical constants.
23. Surface tension : It is defined as the force acting per unit length perpendicular to the line drawn on the surface of liquid. It is denoted by Greek letter γ . It has dimensions of kg s–2 and in SI unit it is expressed as N m–1.
24. Viscosity: It is a measure of resistance to flow which arises due to the internal friction between layers of fluid as they slip past one another while liquid flows. Strong intermolecular forces between molecules hold them together and resist movement of layers past one another. Greater the viscosity, the more slowly the liquid flows. Viscosity of liquids decreases as the temperature rises because at high temperature molecules have high kinetic energy and can overcome the intermolecular forces to slip past one another between the layers.
25. Viscosity coefficient: It is the force when velocity gradient is unity and the area of contact is unit area. Thus “η ‘ is measure of viscosity. SI unit of viscosity coefficient is 1 newton second per square metre (N s m–2) = pascal second (Pa s = 1kgm–1s–1).