Please refer to MCQ Questions Chapter 13 Kinetic Theory Class 11 Physics with answers provided below. These multiple-choice questions have been developed based on the latest NCERT book for class 11 Physics issued for the current academic year. We have provided MCQ Questions for Class 11 Physics for all chapters on our website. Students should learn the objective based questions for Chapter 13 Kinetic Theory in Class 11 Physics provided below to get more marks in exams.
Chapter 13 Kinetic Theory MCQ Questions
Please refer to the following Chapter 13 Kinetic Theory MCQ Questions Class 11 Physics with solutions for all important topics in the chapter.
MCQ Questions Answers for Chapter 13 Kinetic Theory Class 11 Physics
Question. Graph of specific heat at constant volume for a monatomic gas is
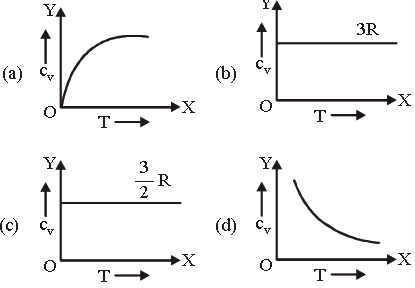
Answer
C
Question. At identical temperatures, the rms speed of hydrogen molecules is 4 times that for oxygen molecules. In a mixture of these in mass ratio H2 : O2 = 1:8, the rms speed of all molecules is n times the rms speed for O2 molecules, where n is
(a) 3
(b) 4/3
(c) (8/3)1/2
(d) (11)1/2
Answer
D
Question. The order of magnitude of the number of nitrogen molecules in an air bubble of diameter 2 mm under ordinary conditions is
(a) 105
(b) 109
(c) 1013
(d) 1017
Answer
D
Question. In Vander Waal’s equation the critical pressure Pc is given by
(a) 3 b
(b) a/27b2
(c) 27a/b2
(d) b2/a
Answer
B
Question. If the potential energy of a gas molecule is U = M/r6 – N/r12, M and N being positive constants, then the potential energy at equilibrium must be
(a) zero
(b) M2/4N
(c) N2/4M
(d) MN2/4
Answer
B
Question. 1 mole of a gas with g = 7/5 is mixed with 1 mole of a gas with g = 5/3, then the value of g for the resulting mixture is
(a) 7/5
(b) 2/5
(c) 24/16
(d) 12/7
Answer
C
Question. Two monatomic ideal gases 1 and 2 of molecular masses m1 and m2 respectively are enclosed in separate containers kept at the same temperature. The ratio of the speed of sound in gas 1 to that in gas 2 is given by

Answer
A
Question. 4.0 g of a gas occupies 22.4 litres at NTP. The specific heat capacity of the gas at constant volume is 5.0JK–1. If the speed of sound in this gas at NTP is 952 ms–1, then the heat capacity at constant pressure is (Take gas constant R = 8.3 JK–1 mol–1)
(a) 7.5 JK–1 mol–1
(b) 7.0 JK–1 mol–1
(c) 8.5 JK–1 mol–1
(d) 8.0 JK–1 mol–1
Answer
D
Question. Air is pumped into an automobile tube upto a pressure of 200 kPa in the morning when the air temperature is 22°C. During the day, temperature rises to 42°C and the tube expands by 2%. The pressure of the air in the tube at this temperature, will be approximately
(a) 212 kPa
(b) 209 kPa
(c) 206 kPa
(d) 200 kPa
Answer
B
Question. The molar heat capacities of a mixture of two gases at constant volume is 13R/6. The ratio of number of moles of the first gas to the second is 1 : 2. The respective gases may be
(a) O2 and N2
(b) He and Ne
(c) He and N2
(d) N2 and He
Answer
C
Question. Work done by a system under isothermal change from a volume V1 to V2 for a gas which obeys Vander Waal’s
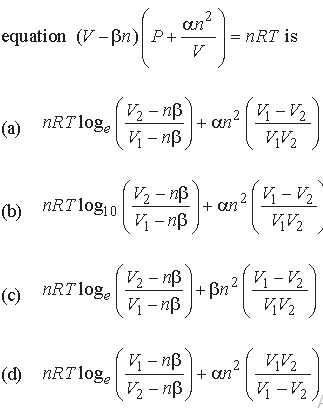
Answer
A
Question. A gas mixture consists of 2 moles of oxygen and 4 moles of Argon at temperature T. Neglecting all vibrational moles, the total internal energy of the system is
(a) 4 RT
(b) 15 RT
(c) 9 RT
(d) 11RT
Answer
D
Question. A graph is plotted with PV/T on y-axis and mass of the gas along x-axis for different gases. The graph is
(a) a straight line parallel to x-axis for all the gases
(b) a straight line passing through origin with a slope having a constant value for all the gases
(c) a straight line passing through origin with a slope having different values for different gases
(d) a straight line parallel to y-axis for all the gases
Answer
C
Question. Four mole of hydrogen, two mole of helium and one mole of water vapour form an ideal gas mixture. What is the molar specific heat at constant pressure of mixture?
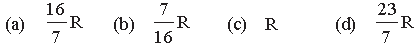
Answer
D
Question. Figure shows a parabolic graph between T and 1/V for a mixture of a gas undergoing an adiabatic process. What is the ratio of Vrms of molecules and speed of sound in mixture?
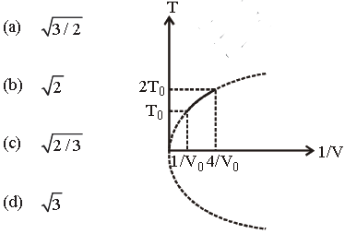
Answer
B
Question. 1 mole of H2 gas is contained in a box of volume V = 1.00 m3 at T =300 K. The gas is heated to a temperature of T = 3000 K and the gas gets converted to a gas of hydrogen atoms. The final pressure would be (considering all gases to be ideal)
(a) same as the pressure initially
(b) 2 times the pressure initially
(c) 10 times the pressure initially
(d) 20 times the pressure initially
Answer
D
Question. In the given (V – T) diagram, what is the relation between pressure P1 and P2 ?
(a) P2 > P1
(b) P2 < P1
(c) Cannot be predicted
(d) P2 = P1
Answer
B
Question. An ideal gas is taken through a process in which the pressure and the volume are changed according to the equation P = kV. Molar heat capacity of the gas for the process is
(a) C = Cv + R
(b) C = Cv + R/2
(c) C = Cv – R/2
(d) C = Cv + 2R
Answer
B
Question. A vessel of volume V contains a mixture of 1 mole of hydrogen and 1 mole oxygen (both considered as ideal). Let f1(v)dv, denote the fraction of molecules with speed between v and (v + dv ) with f2(v)dv, similarly for oxygen. Then,
(a) f1(v) + f2 (v) = f (v) obeys the Maxwell’s distribution law
(b) f1(v), f2(v) will obey the Maxwell’s distribution law separately
(c) neither f1(v), nor f2(v) will obey the Maxwell’s distribution law
(d) f2(v) and f1(v) will be the same
Answer
B
Question. Which one the following graphs represents the behaviour of an ideal gas ?
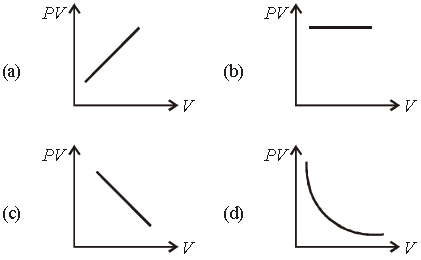
Answer
B
Question. The molar specific heat at constant pressure of an ideal gas is (7/2)R. The ratio of specific heat at constant pressure to that at constant volume is
(a) 5/7
(b) 9/7
(c) 7/5
(d) 8/7
Answer
C
Question. A cubic vessel (with face horizontal + vertical) contains an ideal gas at NTP. The vessel is being carried by a rocket which is moving at a speed of 500 m s–1 in vertical direction. The pressure of the gas inside the vessel as observed by us on the ground
(a) remains the same because 500 ms–1 is very much smaller than vrms of the gas
(b) remains the same because motion of the vessel as a whole does not affect the relative motion of the gas molecules and the walls
(c) will increase by a factor equal to 2 2 2 (vrms + (500) ) / vrms where vrms was the original mean square velocity of the gas
(d) will be different on the top wall and bottom wall of the vessel
Answer
B
Question. 1 mole of an ideal gas is contained in a cubical volume V, ABCDEFGH at 300K (figure). One face of the cube (EFGH) is made up of a material which totally absorbs any gas molecule incident on it. At any given time,
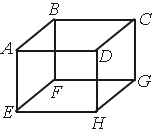
(a) the pressure on EFGH would be zero
(b) the pressure on all the faces will the equal
(c) the pressure of EFGH would be double the pressure on ABCD
(d) the pressure on EFGH would be half that on ABCD
Answer
D
Question. Boyle’s law is applicable for an
(a) adiabatic process
(b) isothermal process
(c) isobaric process
(d) isochoric process
Answer
B
Question. A cylinder containing an ideal gas is in vertical position and has a piston of mass M that is able to move up or down without friction (figure). If the temperature is increased
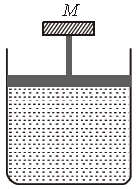
(a) both p and V of the gas will change
(b) only p will increase according to Charles’ law
(c) V will change but not p
(d) p will change but not V
Answer
C
Question. Volume versus temperature graphs for a given mass of an ideal gas are shown in figure. At two different values of constant pressure. What can be inferred about relation between p1 and p2?
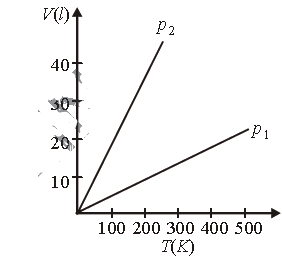
(a) p1 > p2
(b) p1 = p2
(c) p1 < p2
(d) Data is insufficient
Answer
A
Question. N molecules, each of mass m, of gas A and 2 N molecules, each of mass 2 m, of gas B are contained in the same vessel which is maintained at a temperature T. The mean square velocity of molecules of B type is denoted by V2 and the mean square velocity of A type is denoted by V1 then V1/V2 is
(a) 2
(b) 1
(c) 1/3
(d) 2/3
Answer
B
Question. An ideal gas is found to obey an additional law VP2 = constant. The gas is initially at temperature T and volume V. When it expands to a volume 2 V, the temperature becomes
(a) T /√2
(b) 2 T
(c) 2T√2
(d) 4 T
Answer
A
Question. An inflated rubber balloon contains one mole of an ideal gas, has a pressure p, volume V and temperature T. If the temperature rises to 1.1 T, and the volume is increased to 1.05 V, the final pressure will be
(a) 1.1 p
(b) p
(c) less than p
(d) between p and 1.1
Answer
D
Question. A vessel has 6g of hydrogen at pressure P and temperature 500K. A small hole is made in it so that hydrogen leaks out. How much hydrogen leaks out if the final pressure is P/2 and temperature falls to 300 K ?
(a) 2g
(b) 3g
(c) 4g
(d) 1g
Answer
D
Question. The amount of heat energy required to raise the temperature of 1g of Helium at NTP, from T1K to T2K is
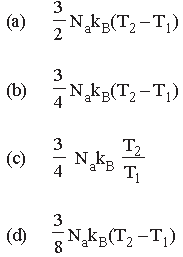
Answer
D
Question. The ratio of the specific heats Cp/Cv = Υ in terms of degrees of freedom (n) is given by
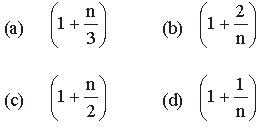
Answer
B
Question. The molecules of a given mass of a gas have r.m.s. velocity of 200 ms–1 at 27°C and 1.0 × 105 Nm–2 pressure. When the temperature and pressure of the gas are respectively, 127°C and 0.05 × 105 Nm–2, the r.m.s. velocity of its molecules in ms–1 is :
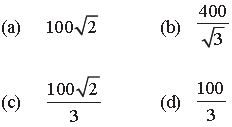
Answer
B
Question. In a vessel, the gas is at a pressure P. If the mass of all the molecules is halved and their speed is doubled, then the resultant pressure will be
(a) 4P
(b) 2P
(c) P
(d) P/2
Answer
B
Question. One mole of an ideal diatomic gas undergoes a transition from A to B along a path AB as shown in the figure.
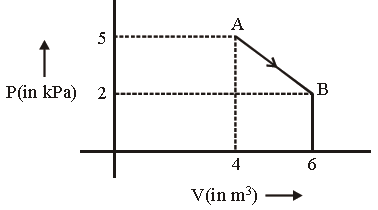
The change in internal energy of the gas during the transition is:
(a) – 20 kJ
(b) 20 J
(c) –12 kJ
(d) 20 kJ
Answer
A
Question. Two vessels separately contain two ideal gases A and B at the same temperature. The pressure of A being twice that of B. Under such conditions, the density of A is found to be 1.5 times the density of B. The ratio of molecular weight of A and B is :
(a) 3/4
(b) 2
(c) 1/2
(d) 2/3
Answer
A
Question. The mean free path of molecules of a gas, (radius ‘r’) is inversely proportional to :
(a) r3
(b) r2
(c) r
(d) r
Answer
B
Question. A gas mixture consists of 2 moles of O2 and 4 moles of Ar at temperature T. Neglecting all vibrational modes, the total internal energy of the system is :-
(a) 15 RT
(b) 9 RT
(c) 11 RT
(d) 4 RT
Answer
C

We hope you liked the above provided MCQ Questions Chapter 13 Kinetic Theory Class 11 Physics with solutions. If you have any questions please ask us in the comments box below.


