See below CBSE Class 12 Chemistry Term 2 Sample Paper Set A with solutions. We have provided CBSE Sample Papers for Class 12 Chemistry as per the latest paper pattern issued by CBSE for the current academic year. All sample papers provided by our Class 12 Chemistry teachers are with answers. You can see the sample paper given below and use them for more practice for Class 12 Chemistry examination.
CBSE Sample Paper for Class 12 Chemistry Term 2 Set A
Section A
1. Complete the following, [Identify ‘X’ and ‘Y’]
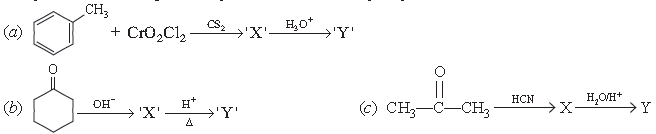
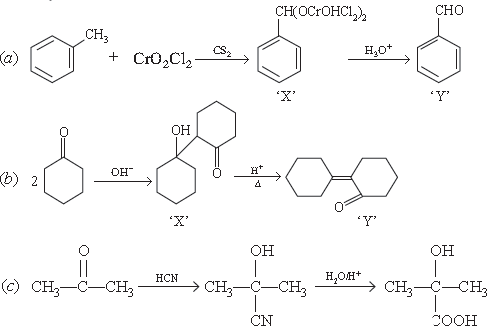
Answer.
2. The following results have been obtained during the kinetic studies of the reaction P + 2Q → R + 2S
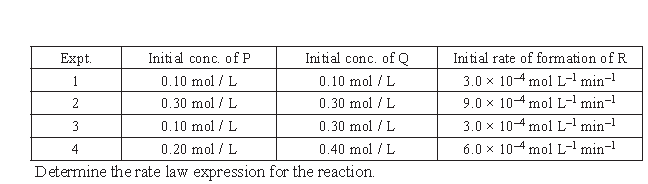
Answer. rate = k [A]x [B]y
3.0 × 10–4 = k [0.1]x [0.10]y …(i)
3.0 × 10–4 = k [0.1]x [0.30]y …(ii)
Dividing (i) by (ii), we get
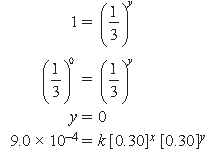
3.0 × 10–4 = k [0.1]x [0.30]y …(iv)
Dividing (iii) by (iv), we get
(3)1 = (3)x
fi x = 1
rate = k [A]1 [B]0
3. Define molar conductivity. On dilution, why does the molar conductivity of HCOOH increase drastically, while that of HCOONa increase gradually?
Answer. Molar conductivity is defined as conducting power of all the ions produced by one molar solution of an electrolyte.
HCOOH is weak electrolyte, on dilution, number of ions as well as mobility of ions increases, hence Ʌm increases drastically HCOONa is strong electrolyte, on dilution number of ions do not increase appreciably only mobility of ions increases hence Ʌm increases gradually.
Section B
4. Write the hybridisation and magnetic behaviour of the following complexes.
(a) [CoF6]3– (b) [Ni(CN)4]2– (c) [Co(NH3)6]3+
[Atomic number of Co = 27, Ni = 28]
Answer. (a) sp3d2, paramagnetic (b) dsp2, diamagnetic (c) d2sp3, diamagnetic
OR
(a) Write IUPAC name of [Mn(H2O)6] SO4.
(b) Why is [Fe(CN)6]4– diamagnetic while [FeF6]3– is paramagnetic?
(c) Why is [Co(en)3]3+ is more stable than [Co(NH3)6]3+?
Answer. (a) Hexaaqua manganese (II) sulphate
(b) [Fe(CN)6]4– does not have unpaired electrons as CN– is strong ligand whereas [FeF6]3– has unpaired electron.
F– is weak ligand, hence paramagnetic.
(c) It is because ‘en’ is didentate ligand forming chelate NH3 is unidentate ligand. Chelates are more stable.
5. How is the rate of reaction affected when
(a) surface area of the reactant is increased (b) temperature of reaction is decreased, and
(c) catalyst is added to reversible reaction?
Answer. (a) Greater the surface area, more will be rate of reaction.
(b) The rate of reaction will decrease.
(c) Catalyst increases the rate of forward as well as backward reaction equally but equilibrium is reached faster in presence of catalyst.
6. (a) Calculate the spin only moment of Co2+ (Z = 27) by writing the electronic configuration of Co and Co2+.
(b) Give reason and select one atom / ion which will exhibit asked property.
(i) Sc3+ or Cr3+ (Exhibit diamagnetic behaviour)
(ii) Cr or Cu (High melting and boiling points)
Answer. (a) Co(27) [Ar]4s23d7
Co2+(27) [Ar]4s03d7
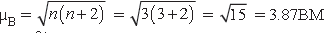
(b) (i) Sc3+ is diamagnetic due to absence of unpaired electron.
(ii) Cr has high melting point. Due to presence of unpaired electrons it forms strong metallic bonds.
7. The electrical resistance of a column of 0.05M KOH solution of length 50 cm and area of cross-section 0.625 cm2
is 5 × 103 ohm. Calculate its resistivity, conductivity and molar conductivity.
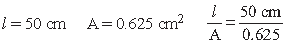
Answer.

8. Give reasons for the following observations.
(a) Physisorption decrease with increase in temperature
(b) Addition of alum purifies water.
(c) Brownian movement provides stability to the colloidal solution.
Answer. (a) It is because physisorption involves weak van der waal’s forces of attraction.
(b) Alum coagulates mud particles settling them down faster.
(c) It causes stirring effect because colloidal particles do not settle down and hence leads to stability.
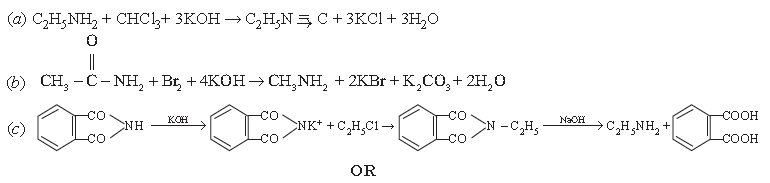
9. Illustrate giving chemical equations for the following.
(a) Carbylamine reaction
(b) Hoffmann bromide reaction
(c) Gabriel phthalimide synthesis
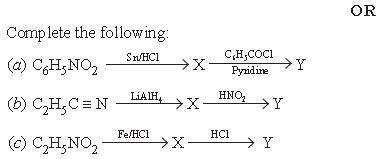
Answer.

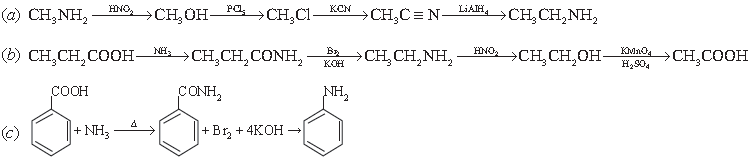
10. How will you carry out following conversions?
(a) Methanamine to ethanamine
(b) Propanoic acid to ethanoic acid
(c) Benzoic acid to aniline
Answer.
Give reason.
(a) pKb of aniline is more than that of methyl aniline.
(b) Methyl aniline in water reacts with ferric chloride to give reddish brown precipitate.
(c) Gabriel phthalimide synthesis cannot be used to prepare aniline.
Answer. (a) Become C6H5 is electron withdrawing, —CH3 is electron releasing
(b) CH3NH2 + H2O æÆ CH3 N+
H3+ OH–, FeCl3 + 3OH– æÆ Fe(OH)3 + 3Cl– reddish brown ppt:
(c) It is because haloareres do not undergo nucleophelic substitution readily.
11. (a) Out of Ti3+, V3+, Cu+, Sc3+, Mn2+, Fe3+ And Cu2+, which ions are coloured in aqueous solution?
[Ti = 22, V = 23, Cr = 24, Fe = 26, Mn = 25, Cu = 29]
(b) Out of Ti2+, V2+, Cr3+, Mn2+ which ion has maximum number of unpaired electrons?
(c) Out of Fe2+ or Mn2+ which is more stable and why?
Answer. (a) Ti3+, V3+, Mn2+, Fe3+, Cu2+ are coloured due to presence of unpaired electrons.
(b) Mn2+ has 5 unpaired electrons.
(c) Mn2+(3d5) is more stable than Fe2+(3d6) due to half filled d-orbitals.
OR
(a) On what ground can you say Sc (21) is transition metal whereas is Zn(30) is not?
(b) Why do translation metals show variable oxidation states?
(c) Name one transition which forms irons in +4 oxidation state. Give reason.
Answer. (a) Sc, [Ar]4s23d1 has incompletely filled d-orbital and therefore it is transition metal. Zn [Ar] 4s23d10 and Zn2+ (3d10) do not have incomplete d-orbitals, hence, it is not transition metal.
(b) It is because electrons from both (n – 1) d and ns take part in bond formation.
(c) Ce4+ because it has stable electronic configuration of xenon.
Section C
12. Read the following passage and answer the questions that follow.
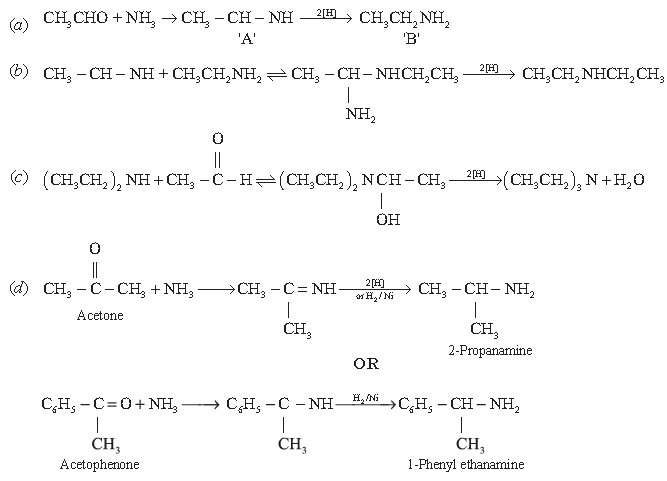
Reductive alkylation is term applied to the process of introducing alkyl groups into ammonia or a primary or secondary amine by means of aldehyde and ketone in presence of reducing agent. The present discussion is limited to those reductive alkylations in which the reducing agent is hydrogen and a catalyst or “nascent” hydrogen usually from a metal acid combination; most of these reductive alkylations have been carried out with hydrogen and catalyst. The principal variation excluded is that in which the reducing agent in formic acid or its derivatives; this modification is known as Leuckart reaction. The process of reductive alkylation of ammonia consist in the addition of NH3 to a carbonyl compound and reduction of addition compound or its dehydration product. The reduction is usually carried out in ethanol solution when the reduction is to be affected catalytically.
Since primary amine is formed in presence of aldehyde, it may react in same manner as ammonia, yielding an addition compound a schiff’s base (R – CH = N – CH2 – R) and finally, secondary amine. Similarly primary amine may react with the imine, forming an addition product which also is reduced to a secondary amine. Finally, the secondary amine may react with either the aldehyde or the imine to give products which are reduced to tertiary amines. Similar reactions may occur when the carbonyl compound employed is a ketone.
(Source: Emerson, W. S. (2011). The Preparation of Amines by Reductive Alkylation. Organic Reactions, 174–255. doi:10.1002/0471264181.or 004.03) (a) Complete the following reaction.
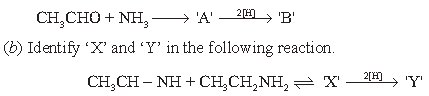
(c) Convert (CH3CH2)2NH to (C2H5)3N using CH3CHO (1)
(d) Convert acetone to 2-propanamine
Answer.