Please refer to Carbon and Its Compound Class 10 Science Important Questions with solutions provided below. These questions and answers have been provided for Class 10 Science based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these problem solutions as it will help them to gain more marks in examinations. We have provided Important Questions for Class 10 Science for all chapters in your book. These Board exam questions have been designed by expert teachers of Standard 10.
Class 10 Science Important Questions Carbon and Its Compound
Very short answer
Question: Why does carbon form compounds mainly by covalent bonding?
Answer: As carbon has four valence electrons and it can neither loose nor gain four electrons thus, it attains noble gas configuration only by sharing of electrons. Thus, it forms covalent compounds.
Question: Write the molecular formula of first two members of homologous series having functional group —Cl.
Answer: The molecular formula of first two members of homologous series having –Cl functional group are CH3Cl and CH3CH2Cl.
Question: Write the molecular formula of the 2nd and 3rd member of the homologous series whose first member is ethene.
Answer: Homologous series of alkenes have general formula, CnH2n whose frst member is ethene.
2nd member of homologous series of alkenes is C3H6 i.e., propene.
3rd member of homologous series of alkenes is C4H8 i.e., butene
Question: Write the molecular formula of first two members of homologous series having functional group —OH.
Answer:The molecular formula of frst two members of homologous series having –OH functional group are CH3OH and CH3CH2OH.
Question: Write the molecular formula of the 2nd and 3rd member of the homologous series whose first member is methane.
Answer: Methane, CH4 is an alkane. Alkanes have general formula, CnH2n+2.
2nd member of homologous series of alkanes is C2H6 i.e., ethane.
3rd member of homologous series of alkanes is C3H8 i.e., propane.
Question: Why are covalent compounds generally poor conductors of electricity?
Answer: Covalent compounds are generally poor conductors of electricity because they do not have free electrons or ions.
Question: Write the name and formula of the 2nd member of homologous series having general formula CnH2n.
Answer: Homologous series of alkenes have general formula, CnH2n whose first member is ethene. 2nd member of homologous series of alkenes is C3H6 i.e., propene. 3rd member of homologous series of alkenes is C4H8 i.e., butene.
Question: Write the name and formula of the 2nd member of homologous series having general formula CnH2n+2.
Answer: Methane, CH4 is an alkane. Alkanes have general formula, CnH2n+2.
2nd member of homologous series of alkanes is C2H6 i.e., ethane.
3rd member of homologous series of alkanes is C3H8 i.e., propane.
Question: Write the name and formula of the 2nd member of homologous series having general formula CnH2n + 2.
Answer:

Question: Write the number of covalent bonds in the molecule of ethane.
Answer: The structural formula of ethane (C2H6) is :
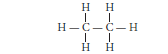
There are total 7 covalent bonds. Six C — H covalent bonds and one C — C covalent bond
Question: Write the number of covalent bonds in the molecule of butane, C4H10.
Answer: Butane (C4H10) has the following structural formula as :

Total number of covalent bonds is 13 in which 10 C — H and 3 C – C covalent bonds.
Question: Write the name of each of the following functional groups :
(a) — OH (b)- C-
ll
O
Answer:
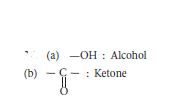
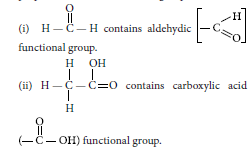
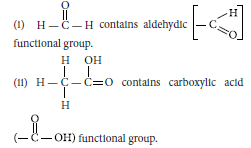
Question: Defne the term functional group. Identify the functional group present in
(i) H C H
O
(ii) H C C O
H
H
OH
Answer: An atom or a group of atoms present in a molecule which largely determines its chemical properties, is called functional group.
Question: Name the functional group present in each of the following organic compounds :
(i) C2H5Cl
(ii) C2H5OH
Answer: (i) C2H5Cl contains —Cl (chloro) group which belongs to halo functional group.
(ii) C2H5OH contains —OH group which belongs to alcoholic functional group.
Question: Write the name and formula of the second member of the carbon compounds having functional group –OH.
Answer: Those having —OH as functional group belong to alcohol family. Second member of this family is ethanol, C2H5OH.
Question: Write the name and formula of the first member of the series of carbon compounds
O
ll
having functional group — C— OH
Answer: Carbon compound containing group is called carboxylic acid.THe first member
of this family is methanoic acid (HCOOH).

Short Questions type Answer: I
Question: What happens when 5% alkaline KMnO4 solution is added drop by drop to warm ethanol taken in a test tube? State the role of alkaline KMnO4 solution in this reaction.
Answer: When 5% alkaline KMnO4 solution is added drop by drop to warm ethanol then it gets oxidised
to ethanoic acid.
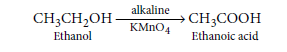
Here, alkaline KMnO4 acts as an oxidising agent i.e., the substance which is capable of adding oxygen to others. Thus, alkaline KMnO4 provides oxygen to ethanol to form ethanoic acid.
Question: State two properties of carbon which lead to a very large number of carbon compounds.
Answer: Carbon forms a large number of carbon compounds like long chains which may be straight
or branched chains or ring of different sizes due to its tetravalency and unique property of catenation.
Carbon due to its small size forms exceptionally stable compounds by forming strong bonds.
Question. An organic compound ‘P’ is a constituent of wine. ‘P’ on reacting with acidified K2Cr2O7 forms another compound ‘Q’. When a piece of sodium is added to ‘Q’ a gas ‘R’ evolves which burns with a pop sound.
Identify P, Q and R and write the chemical equations of the reactions involved.
Answer:
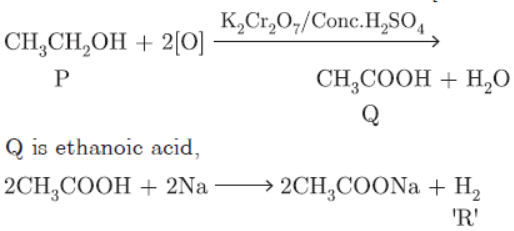
Question. a. Define the term functional group. Identify the functional group present in b. What happens when 5% alkaline KMnO4 solution is added drop by drop to warm ethanol taken in a test tube? State the role of alkaline KMnO4 solution in this reaction.
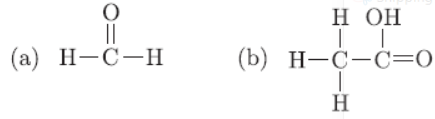
Answer:
a. Functional group is an atom or group of atoms which determine chemical properties of organic compounds. (a) Aldehyde, (b) Carboxylic acid.
b. Ethanoic acid is formed.
CH3 CH2 OH + 2[O] 5% alkaline → CH3 COOH
KMnO4
KMnO4 acts as oxidising agent.
Question. Give reasons for the following observations:
a. The element carbon forms a very large number of compounds.
b. Air holes of a gas burner have to be adjusted when the heated vessels get blackened by the flame.
c. Use of synthetic detergents causes pollution of water.
Answer:
a. It is due to tetravalency of carbon and property of catenation shown by carbon to maximum extent.
b. Air holes must be kept open fully so that complete combustion of fuel takes place producing blue flame.
c. Some of detergents are not bio¬degradable, they create water pollution.
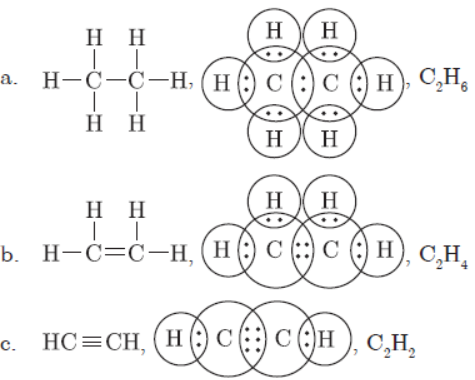
Question. Write the molecular formula of the following compounds and draw their electron dot structures:
(a) Ethane (b) Ethene (c) Ethyne
Answer:
Question. What are covalent compounds? Why are they different from ionic compounds? List three characteristic properties.
Answer:Ans : Those compounds in which bonds are formed by sharing of electrons are covalent compounds.
While ionic compounds are formed by complete transfer of electrons.
Question. A compound X on heating with excess of cone. H2SO4 at 443 K gives an unsaturated compound Y. X also reacts with sodium metal to evolve a colourless gas Z. Identify X, Y and Z. Write the equations of the chemical reaction of formation of Y and also write the role of conc. sulphuric acid in the reaction.
Answer: X is CH3CH2OH, Ethanol Y is Ethene, Z is H2.
C2H5OH Cone.H2SO4 → CH2 = CH2+H2O
X 443K Y
2CH3 CH2 OH + 2Na → 2CH3 CH3 ONa + H2z
Ethanol Sodium ethoxide
Cone. H2SO4 acts as dehydrating agent.
Question. Write any three physical properties and three uses of ethanol.
Answer:
Properties
a. Ethanol has specific smell.
b. It is soluble in water.
c. It has burning taste.
Uses
a. It is used as solvent.
b. It is used as an antiseptic.
c. It is used in wine, beer, whisky, etc.
d. It is used for preparation of ethanoic acid, ethyl ethanoate (esters).
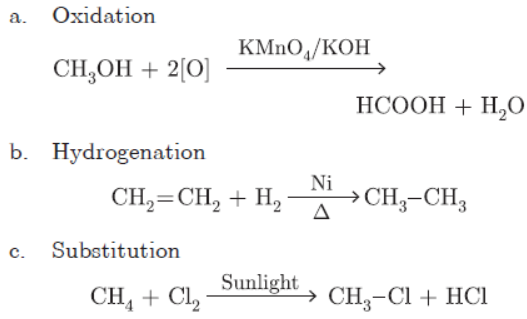
Question. Explain the following reactions with chemical equations:
a. Oxidation
b. Hydrogenation
c. Substitution
Answer:
Question. Under which condition an oxidation reaction can be called as combustion reaction. Illustrate your answer with example.
Answer: When oxidation reaction produces heat as well as light it is called combustion reaction.
CH4 + 2O2 → CO2 + 2H2O + heat + light
It is oxidation as well as combustion reaction.
Question. What is an oxidising agent? What happens when oxidising agent is added to propanol? Explain with the help of a chemical equation.
Answer: Oxidising agent is a substance which adds oxygen or remove hydrogen. Propanol will get oxidised to propanoic acid.
CH3CH2CH2OH + 2(O) Alkaline KMnO4 →
CH3CH2COOH+ H2O
Question. C3H6, C4H8 and C5H10 belong to same 1 homologous series.
a. Define homologous series.
b. Why the melting and boiling point of C5H10 is higher than C4H3?
c. Arrange these hydrocarbons in order of increasing boiling points.
Answer:
a. The series of organic compounds having same functional group and similar chemical properties is called homologous series.
b. C5H10 has higher molecular mass, more surface area, more van der Waal’s forces of attraction, hence higher boiling point than C4H8.
c. C3H6 < C4H8 < C5H10
Question. a. Why are covalent compounds generally poor conductors of electricity?
b. Name the following compound:
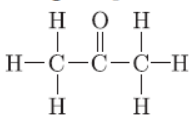
c. Name the gas evolved when ethanoic acid is added to sodium carbonate. How will you test the presence of this gas.
Answer:
a. It is because they do not form ions in their aqueous solution.
b. Propanone
c. Carbon dioxide gas will be liberated. Test: Pass the gas through lime water. If lime water turns milky it shows the presence of CO2 gas.
Question. What is meant by isomers? Draw the structures of two isomers of butane, C4H10. Explain why we cannot have isomers of first three members of alkane series.
Answer: Isomers are those compounds which have same molecular formula but different structural formula.

Question. The general formula of three compounds A, B and C is Cn (H2n . ‘B’ has highest boiling point and ‘C’ has lowest boiling point.
a. Mention the type of compounds A, B, C.
b. Which of these have minimum number of carbon atoms?
c. Name the homologous series to which A, B and C belong.
Answer:
a. A, B, C are unsaturated compounds.
b. C has minimum number of carbon atoms..
c. They belong to alkene homologous series.
Question. The structural formula of an ester is

Write the structural formula of the acid and the alcohol from which it might be prepared. Name the process of formation of an ester.
Answer:

Esterification is the process of forming ester
Question. Write the name and general formula of a chain of hydrocarbons in which an addition reaction with hydrogen is possible. State the essential conditions for an addition reaction. Stating this condition, write a chemical equation giving the name of the reactant and the product of the reaction.
Answer: Alkenes CnH2n Alkynes CnH2n-2
In above two series of hydrocarbons, addition of H2 is possible.
Hydrogen is added in presence of nickel as catalyst and heating is needed.
CH2= CH2 + H2 Ni → heat CH3 – CH3
Ethene Hydrogen Ethene
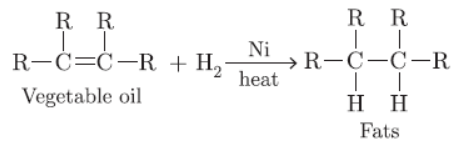
Question. Why should we prefer vegetable oils over animal fats for cooking food? Give a balanced chemical equation for reaction of hydrogenation of vegetable oils. Name the catalyst in the reaction.
Answer: Vegetable oils are unsaturated and do not lead to formation of cholesterol. Animals fats are saturated lead to formation of cholesterol which can be deposited in arteries.
Question. Compare the structures of benzene and cyclohexane by drawing them.
Answer:

Benzene has 3 double bonds whereas cyclohexane has all single bonds.
Question. a. Give chemical tests to detect the presence of (a) ethanol (b) ethanoic acid.
b. Why ethanoic acid is called glacial acetic acid?
Answer:
(i) (a) Ethanol reacts with Na to liberate
H2 gas
2CH3CH2OH 2Na 2CH3CH2ONa H2 + → +
(b) Ethanoic acid gives brisk effervescence of CO2 with NaHCO3
CH3COOH NaHCO3+ →
CH3COONa + H2O + CO2
(ii) It is because pure acetic acid (anhydrous) solidifies into solid crystals just below the room temperature at 16.7°C and look like glacier of snow.
Question. Convert CH4 into CC14 by substituting hydrogen atom with chlorine atom in successive reactions. Why this reaction is referred to as substitution reaction.
Answer:
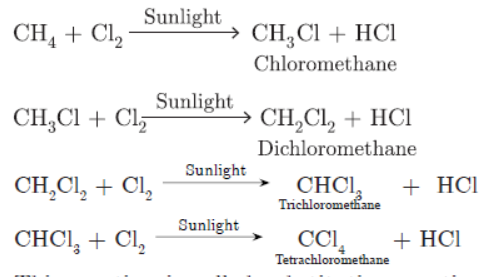
This reaction is called substitution reaction because hydrogen atom is being substituted by ‘Cl’ in each step.
Question. Why is homologous series of carbon compounds so called? Write the chemical formula of two consecutive members of any two homologous series and state the part of these compounds that determines their (a) physical and (b) chemical properties.
Answer: Homologous means members of same family that is why series of carbon compounds having same functional group and similar properties is called homologous series.
Homologous series of alcohol.
CH3OH Methanol
C2H5OH Ethanol
CH3— and C2H5— groups determine physical
properties and —OH group determines chemical properties. Homologous series of aldehyde.
CH3OH Ethanol
C2H5OH Propanal
Here, CH3—and C2H5— groups determine physical properties while —CHO group determines chemical properties.
Question. What is meant by homologous series of carbon compounds? Write the general formula of (i) alkenes, and (ii) alkynes. Draw the structures of the first member of each series to show the bonding between the two carbon atoms.
Ans : The series of organic compounds having same functional group and similar chemical properties is called homologous series.
i. Alkenes CnH2n
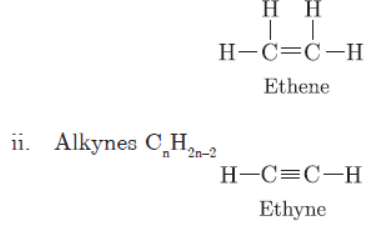
Question. Write the chemical equations to show what happens when
a. an ester reacts with a base?
b. methane is treated with chlorine in the presence of sunlight?
c. ethanol reacts with ethanoic acid in the presence of sulphuric acid?
Ans :
a. CH3COOC2H5 + NaOH →
CH3COONa + C2H5OH
b. CH4 + Cl2 Sunlight → CH3Cl + HCl
c. CH3COOH + C2H5OH Conc.H2SO4 →
CH3COOC2H5 + H2O
Question. Write the respective chemical equations to show what happens when
a. methane is burned in presence of oxygen?
b. ethanol is heated with concentrated sulphuric acid at 443 K?
c. ethanol reacts with ethanoic acid in the presence of an acid acting as a catalyst?
Answer:
a. CH4 + 2O2 → CO2 + 2H2O
b. CH3CH2OH Conc.H2SO4 → 443k CH2=CH2 + H2O
c. CH3COOH + C2H5OH Conc.H2SO4 →
CH3COOC2H5 + H2O
Question. Write chemical equations to describe two different oxidations of ethanol. List two uses of ethanol.
Answer: CH3CH2OH + 2[O] K2Cr2O7/H2SO4 → CH3COOH
CH2CH2OH + O2 → CO2 + H2O + Heat + Light
Ethanol is used as an antiseptic and solvent
Question. What is difference between the molecule of soaps and detergents, chemically? Explain the cleansing action of soaps.
Answer: Soaps are sodium or potassium salts of fatty acids.
They contain —COONa group. Detergents are sodium or potassium salts of sulphonic acids. They contains —SO3Na or —SO4Na group. Soap has ionic end which is hydrophilic, interacts with water while carbon chain is hydrophobic interacts with oil, grease. The soap molecules orient themselves in a cluster in which hydrophobic tails are inside the cluster and ionic ends
face outside. These cluster are called micelles. These attract oil which is washed away by water.
Question. a. What is meant by a functional group in an organic compound? Name the functional group present in
(1) CH3CH2OH
(2) CH3COOH
b. State one point of difference between soap and synthetic detergent.
Answer:
a. Functional group is an atom or group of atoms which determine chemical properties of organic compounds. (1) Alcohol, (2) Carboxylic acid.
b. Soaps do not work well with hard water as form insoluble scum whereas detergents work well with hard water.
Question. A carbon compound X turns blue litmus to red and has a molecular formula C2H4O2. Identify X and draw its structure. Write chemical equation for the reaction and name of the product formed in each case when X reacts with
a. ethanol in the presence of concentrate H2SO4.
b. sodium carbonate.
Answer: 149
X is CH3COOH
Question. What are esters? How are they prepared? List two uses of esters.
Answer: Esters are pleasant fruity smelling compounds with general formula R—COOR’. They are prepared by reaction of carboxylic acid and alcohol in presence of cone. H2SO4.
CH3COOH + C2H5OH Conc.H2SO4 →
CH3COOC2H5 + H2O
Uses
i. They are used in cold drinks and ice creams as synthetic flavours.
ii. They are used in perfumes.
Question. State the meaning of functional group in an organic compound. Write the formula of the functional group present in alcohols, aldehydes, ketones and carboxylic acids.
Answer: Functional group is an atom or group of atoms which determine chemical properties of organic compounds (a) Aldehyde, (b) Carboxylic acid.

Question. A carboxylic acid (molecular formula C2H4O2) reacts with an alcohol in the presence of an acid catalyst to form a compound X. The alcohol on oxidation with alkaline KMnO4 following by acidification gives the same carboxylic acid C2H4O2. Write the name and structure of (i) carboxylic acid, (ii) alcohol and (iii) the compound ‘X’.
Answer: CH3COOH + C2H5OH Conc.H2SO4 →
Ethanoic acid Ethanol
CH3COOC2H5 + H2O
CH3CH2OH + 2[O] KMnO4/KOH → X
CH3COOK
H+ → CH3COOH + K+
X is CH3COOC2H5, ethyl ethan
Question: Write a chemical equation to represent what happens when hydrogen gas is passed through an unsaturated hydrocarbon in the presence of nickel acting as a catalyst.
Answer:

Short Questions type Answer: II
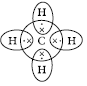
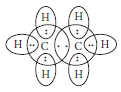
Question: What are covalent bonds? Show their formation with the help of electron dot structure of methane. Why are covalent compounds generally poor conductors of electricity?
Answer: Covalent bonds are those bonds which are formed by sh aring the valence electrons between two atoms. Electron dot structure of methane is shown in the figure. Covalent compounds are generally poor conductors of electricity because they do not have free electrons or ions.
Question: Give reasons for the following :
(i) Element carbon forms compounds mainly by covalent bonding.
(ii) Diamond has high melting point.
(iii) Graphite is a good conductor of electricity.
Answer:(i) As carbon has four valence electrons and it can neither loose nor gain four electrons thus, it attains noble gas configuration only by sharing of
electrons. Thus, it forms covalent compounds.
(ii) In diamond, each carbon atom is bonded to four other carbon atoms forming a rigid three-dimensional structure. This makes diamond the hardest known substance. Thus, it has high melting point.
(iii) In graphite, each carbon atom is bonded to three other carbon atoms by covalent bonds in the same plane giving a hexagonal array. Thus, only three valence electrons are used for bond formation and hence, the fourth valence electron is free to move. As a result, graphite is a good conductor of electricity.
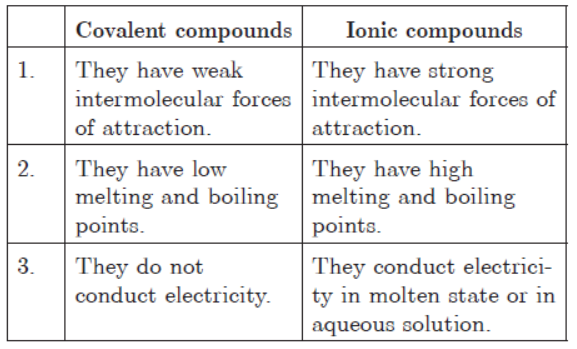
Question: Distinguish between ionic and covalent compounds under the following properties :
(i) Strength of forces between constituent elements.
(ii) Solubility of compounds in water.
(iii) Electrical conduction in substances.
Answer: (i) Ionic compounds have strong forces between its constituent elements because they are held together by strong electrostatic forces of
attraction. On the other hand, covalent compounds have weaker forces of attraction between its constituent elements as bond is formed by sharing
of electrons.
(ii) Ionic compounds are highly soluble in water but they are insoluble in non-polar organic solvents. On the other hand, covalent compounds
are generally insoluble in water but they are soluble in non-polar solvents.
(iii) Ionic compounds conduct electricity in aqueous state or in molten state because they produce ions which are good conductors of electric
current. On the other hand, covalent compounds are bad conductors of electricity due to absence of ions.
Question: What are covalent compounds? Why are they difierent from ionic compounds? List their three characteristic properties.
Answer:Covalent compounds are those compounds which are formed by sharing of valence electrons between the atoms e.g., Hydrogen molecule is formed by mutual sharing of electrons between two hydrogen atoms.
They are different from ionic compounds as ionic compounds are formed by the complete transfer of electrons from one atom to another e.g., NaCl is formed when one valence electron of sodium gets completely transferred to outer shell of chlorine atom.
The characteristic properties of covalent compounds are :
(i) They are generally insoluble or less soluble in water but soluble in organic solvents.
(ii) They have low melting and boiling points.
(iii) They do not conduct electricity as they do not contain ions.
Question: What is meant by isomers? Draw the structures of two isomers of butane, C4H10. Explain why we cannot have isomers of first three members of alkane series.
Answer: Isomers are those molecules which have the same molecular formula but different structural formular i.e., show different properties.
The structures of possible isomers of butane (C4H10) are :
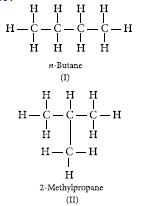
the FIrst three members of alkane series are :
(i) CH4 (methane) (ii) C2H6 (ethane)
(iii) C3H8 (propane)
In the above members of alkane series, it is not possible to have different arrangements of carbon atoms. Thus, we cannot have isomers of frst three
members of alkane series.
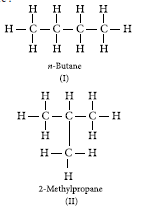
Question: An aldehyde as well as a ketone can be represented by the same molecular formula, say C3H6O. Write their structures and name them. State the relation between the two in the language of science.
Answer: The aldehyde and ketone represented by the molecular formula, C3H6O
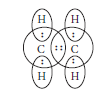
Question: Write the molecular formula of the following compounds and draw their electron-dot structures :(i) Ethane (ii) Ethene(iii) Ethyne
Answer: (i) Molecular formula of ethane is C2H6. Its electron dot structure is :
(ii) Molecular formula of ethene is C2H4.Its electron dot structure is :
(iii) Molecular formula of ethyne is C2H2. Its electron dot structure is :
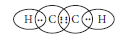
Question: Why is homologous series of carbon compounds so called? Write the chemical
formula of two consecutive members of any homologous series and state the part of these compounds that determines their (i) physical and (ii) chemical properties.
Answer: A homologous series is the family of organic compounds having the same functional group, similar chemical properties but the successive (adjacent) members of the series are differ by a CH2 unit or 14 mass units.
Consecutive members of the homologous series of alcohols are :
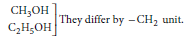
the physical properties are determined by alkyl group/hydrocarbon part/part other than the functional group. the chemical properties are determined by
functional group such as —OH group.
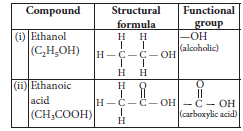
Question: State the meaning of functional group in a carbon compound. Write the functional group present in (i) ethanol and (ii) ethanoic acid and also draw their structures.
Answer:
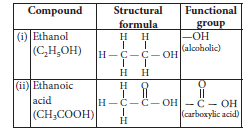
Question: State the meaning of the functional group in an organic compound. Write the formula of the functional group present in alcohols, aldehydes, ketones and carboxylic acids
Answer:
Question: Define the term ‘structural isomerism’. Explain why propane cannot exhibit this property. Draw the structures of possible isomers of butane, C4H10.
Answer: Two or more organic compounds having the same molecular formula but different structures, are called structural isomers and the phenomenon is known as structural isomerism.
There is no possible isomers for propane as it contains three carbon atoms and it is not possible to have different arrangements of these carbon atoms.
Isomers are those molecules which have the same molecular formula but different structural formular i.e., show different properties.The structures of possible isomers of butane (C4H10) are :
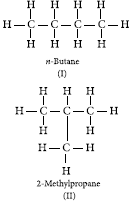
The first three members of alkane series are :
(i) CH4 (methane) (ii) C2H6 (ethane)
(iii) C3H8 (propane)
In the above members of alkane series, it is not possible to have different arrangements of carbon atoms. Thus, we cannot have isomers of first three members of alkane series.
Question: Two carbon compounds X and Y have the molecular formula C4H8 and C5H12 respectively. Which one of these is most likely to show addition reaction? Justify your answer. Also give the chemical equation to explain the process of addition reaction in this case.
Answer: All unsaturated hydrocarbons (containing double or triple bonds) have tendency to get converted to saturated hydrocarbons (single bonds) by adding small molecules such as hydrogen (H2), halogens (X2), etc. Such reactions are called addition reactions. Compound X i.e. C4H8 belongs to alkene series (CnH2n) while compound Y i.e. C5H12 belongs to alkane series (CnH2n + 2). Thus, compound X will undergo addition reaction.

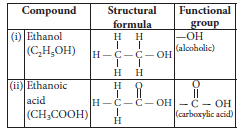
Question: What is meant by functional group in carbon compounds? Write in tabular form the structural formula and the functional group present in the following compounds :
(i) Ethanol
(ii) Ethanoic acid
Answer: An atom or a group of atoms present in a molecule which largely determines its chemical properties, is called functional group.