Please refer to Aldehydes Ketones and Carboxylic Acids Class 12 Chemistry Important Questions with solutions provided below. These questions and answers have been provided for Class 12 Chemistry based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these problem solutions as it will help them to gain more marks in examinations. We have provided Important Questions for Class 12 Chemistry for all chapters in your book. These Board exam questions have been designed by expert teachers of Standard 12.
Class 12 Chemistry Important Questions Aldehydes Ketones and Carboxylic Acids
Very Short Answer Questions
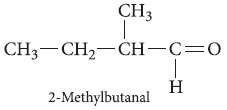
Question. Write the structure of 2-methylbutanal.
Answer.
Question. Give a simple chemical test to distinguish between the following pair of compounds:
CH3CH2CHO and CH3CH2COCH3
Answer. Propanal and propanone can be distinguished by their reactions with Tollens’ reagent.
Propanal will form the silver mirror, but propanone does not react.
Question. Name the reagents used in the following reactions :

Answer.

Question. Give reason :
Aldehydes are more reactive than ketones towards nucleophilic reagents.
Answer. Ketones are less reactive than aldehydes towards nucleophilic addition reactions because :
The two electron releasing alkyl groups decrease the magnitude of positive charge on carbonyl carbon and make it less susceptible to nucleophilic attack.

The two bulkier alkyl groups hinder the approach of the nucleophile to the carbonyl carbon. This is called steric factor.
Question. Arrange the following compounds in an increasing order of their reactivity in nucleophilic addition reactions :
ethanal, propanal, propanone, butanone.
Answer. Butanone < propanone < propanal < ethanal.
Question. Give chemical tests to distinguish between Benzophenone and acetophenone
Answer. Acetophenone and benzophenone can be distinguished by iodoform test.
Acetophenone will give the yellow precipitate of iodoform, but benzophenone will not react.
Question. Arrange the following compounds in an increasing order of their property as indicated :
Acetaldehyde, acetone, methyl tert-butyl ketone (reactivity towards HCN)
Answer.

Question. Write the structure of the product formed in the following reaction :
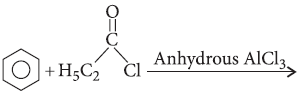
Answer.
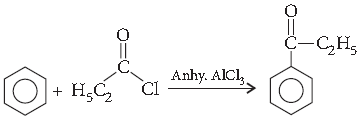
Question. Predict the organic product of the following reactions :
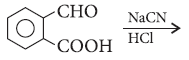
Answer.

Question. Predict the products of the following reactions:

Answer.
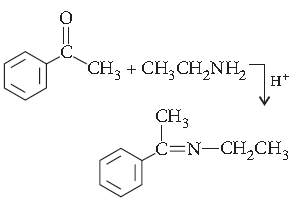
Question. What is Tollens’ reagent? Write one usefulness of this reagent.
Answer. Tollens’ reagent is an ammoniacal silver nitrate solution.
Tollens’ reagent is used to test an aldehyde. Both aliphatic and aromatic aldehydes reduce Tollens’ reagent and give silver mirror.
Question. Describe how the following conversions can be brought about :
Cyclohexanol to cyclohexan 1-one
Answer.

Question. Illustrate the following name reaction :
Wolf–Kishner reduction reaction
Answer. Wolf-Kishner reduction reaction : The carbonyl group of aldehydes and ketones is reduced to CH2 group on treatment with hydrazine followed by heating with potassium hydroxide in a high boiling solvent such as ethylene glycol.

Question. Write chemical equations to illustrate the following name bearing reaction :
Cannizzaro’s reaction.
Answer. Cannizzaro’s reaction : Aldehydes which do not contain a-H atom undergo disproportionation when heated with concentrated (50 %) NaOH.

Question. State chemical tests to distinguish between the following pairs of compounds :
Propanal and propanone
Answer. Propanal reduces Tollen’s reagent into silver mirror while propanone does not gives this test
CH3CH2CHO + 2[Ag(NH3)2]+ + 2OH–→
Propanal Tollens’ reagent
CH3CH2COONH4 + 2Ag + H2O + 2NH3
Silver mirror
Question. Write the IUPAC name of the compound :

Answer.

Question. Write the IUPAC name of the following :

Answer. Hex-2-en-4yn-oic acid
Question. Write the IUPAC name of

Answer. 3-Bromo-5-chloro Benzoic acid
Question. Write the IUPAC name of the following compound:

Answer. Ethyl-4-chlorobenzoate
Question. Name the reagents used in the following reaction :
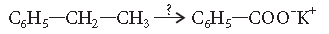
Answer. Alkaline potassium permanganate (KMnO4, KOH)
Question. How will you convert the following :
Ethanal to 2-hydroxy propanoic acid
Answer.

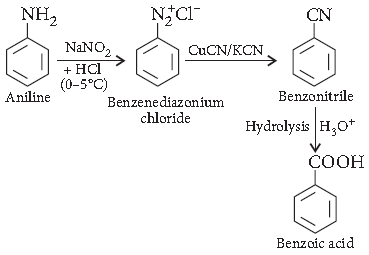
Question. How will you obtain the following :
Benzoic acid from Aniline
Answer.
Question. Predict the organic products of the following reaction :
Answer.
Question. Why carboxylic acid does not give reactions of carbonyl group?
Answer. The carbonyl group in — COOH is inert and does not show nucleophilic addition reaction like carbonyl compound. It is due to resonance stabilisation of carboxylate ion :
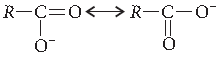
Question. Distinguish between
CH3COOH and HCOOH
Answer. Add Tollens’ reagent to formic acid and warm.
Silver mirror is formed.
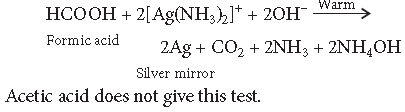
Question. Predict the products of the following reaction :

Answer.
Question. Name the reagent used in the following reaction :

Answer.

Question. Describe the following giving chemical equation :
De-carboxylation reaction
Answer. Decarboxylation : Sodium or potassium salt of carboxylic acids on heating with soda lime (NaOH and CaO), loses a molecule of carbon dioxide and alkanes are obtained as products.
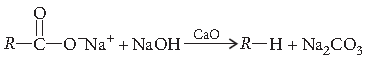
Question. How will you bring about the following conversion?
Benzoic acid to Benzaldehyde
Answer.

Question. Account for the following :
Carboxyl ic acids do not give reactions of carbonyl group.
Answer. In carboxylic acid C = O is in resonance and not available for reaction.
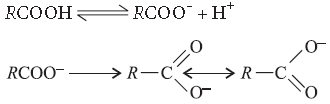
Question. Give simple chemical tests to distinguish between the following pairs of compounds :
Benzoic acid and Phenol
Answer. Phenol and benzoic acid can be distinguished by their reactions with sodium bicarbonate solution. Benzoic acid will give effervescence with NaHCO3 but phenol will not react.
Question. Give simple chemical tests to distinguish between the following pairs of compounds:
Benzoic acid and Ethyl benzoate
Answer. Benzoic acid and ethyl benzoate can be distinguished by their reactions with sodium bicarbonate solution. Benzoic acid will give effervescence with NaHCO3.
Question. Write the chemical equation to illustrate the following name reaction :
Hell-Volhard-Zelinsky reaction
Answer. Hell-Volhard-Zelinsky reaction : Carboxylic acids react with chlorine or bromine in the presence of phosphorous to give compounds in which a-hydrogen atom is replaced by halogen atom.

Question. Give reasons :
Chloroacetic acid is stronger than acetic acid.
Answer. Chloroacetic acid has lower pKa value than acetic acid; ‘Cl’ in chloroacetic acid shows –I effect, it creates less electron density on oxygen of carboxylic acid. Thus, release of proton becomes easier. In case of acetic acid, the state of affair is just opposite. Hence, chloroacetic acid is stronger than acetic acid.
Question. Predict the products of the following reaction :

Answer.

Question. Arrange the following compounds in increasing order of their acid strengths
(CH3)2CHCOOH, CH3CH2CH(Br)COOH,
CH3CH(Br)CH2COOH
Answer. We know that + I-effect decreases while –I-effect increases the acid strength of carboxylic acids. The overall acid strength increases in the order.
(CH3)2CHCOOH < CH3CH(Br)CH2COOH < CH3CH2CH(Br)COOH
Question. How are the following conversions carried out Acetic acid to methylamine.
Answer. Acetic acid to methyl amine :

Question. Write one chemical equation for each to illustrate the following reaction :
Fischer esterification.
Answer. Fischer esterification :
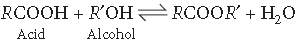
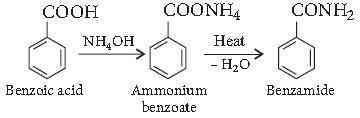
Question. How would you convert :
Benzoic acid to benzamide.
Answer. Benzoic acid to benzamide :
Short Answer Questions
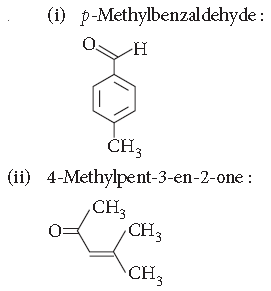
Question. Draw the structures of the following :
(i) p-Methylbenzaldehyde
(ii) 4-Methylpent-3-en-2-one
Answer.
Question. Write the structure of A and B in the following reaction :
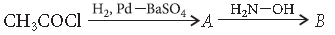
Answer.

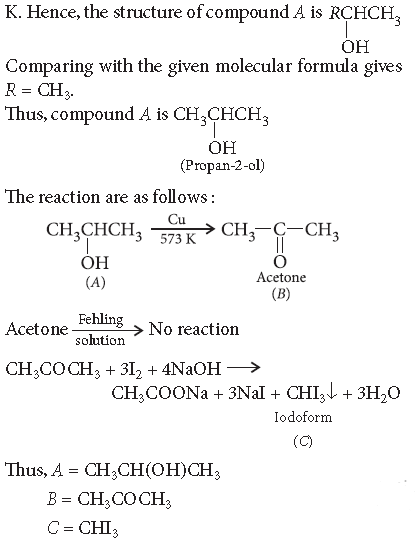
Question. An organic compound A, having the formula, C3H8O, on treatment with copper at 573 K, gives B. B does not reduce Fehling’s solution but gives a yellow precipitate of the compound C with I2/NaOH. Deduce the structure of A, B and C.
Answer. Compound B gives positive iodoform test, it means it contains —COCH3 (methyl ketone) group i.e., it is a ketone. Moreover, B is obtained by the oxidation of A, thus A must be a 2° alcohol. (As only 2° alcohol give ketones on oxidation with Cu at 573
Question. Write the main product in the following equations :
Answer.
Question. 67

Answer.

Question. Predict the products of the following reactions :
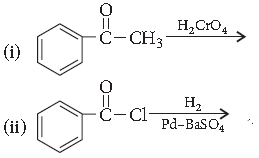
Answer.

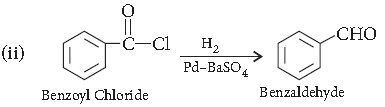
Question. Explain the mechanism of a nucleophilic attack on the carbonyl group of an aldehyde or a ketone.
Answer. Mechanism of nucleophilic addition reactions :
Nucleophile attacks from the top face :

A nucleophile attacks the electrophilic carbon atom from a direction perpendicular to the plane of sp2 hybridised orbital of carbonyl carbon. The hybridisation of carbon changes from sp2 to sp3 in this process and a tetrahedral alkoxide intermediate is produced. The intermediate captures a proton from the reaction medium to give the electrically neutral product. The net result is addition of Nu– and H+ across the carbon oxygen double bond.
Question. (a) Write the product of the following reaction :

(b) Give simple chemical tests to distinguish between the following pairs of compounds :
Benzaldehyde and benzoic acid
Answer.
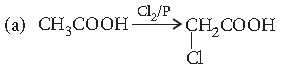
(b) Benzaldehyde when treated with ammoniacal silver nitrate gives silver mirror.
C6H5CHO + 2[Ag(NH3)2]+ 3OH– →
C6H5COO–+ 2Ag + 4NH3 + 2H2O
Siliver mirror
Benzoic acid reacts with sodium bicarbonate to liberate CO2.

Question. Describe how the following conversions can be brought about :
(i) Ethylbenzene to benzoic acid
(ii) Bromobenzene to benzoic acid
Answer.

Question. Arrange the following compounds in an increasing order of their indicated property :
(i) Benzoic acid, 4-Nitrobenzoic acid, 3,
4-Dinitrobenzoic acid.
4-Methoxybenzoic acid (acid strength)
(ii) CH3CH2CH(Br)COOH,
CH3CH(Br)CH2COOH
(CH3)2CHCOOH, CH3CH2CH2COOH
(acid strength)
Answer. (i) 4-methoxybenzoic acid < benzoic acid < 4-nitrobenzoic acid < 3,4-dinitrobenzoic acid.
(ii) The overall acidic strength increases in the order :
(CH3)2CHCOOH < CH3CH2CH2COOH < CH3CH(Br)CH2COOH < CH3CH2CH(Br)COOH
Question. State reasons for the following :
(i) Monochloroethanoic acid has a higher pKa value than dichloroethanoic acid.
(ii) Ethanoic acid is a weaker acid than benzoic acid.
Answer. (i) The strength of an acid is indicated by pKa value, where, pKa = – log Ka Since monochloroethanoic acid is weaker than dichloroethanoic acid so it has lower value of dissociation constant Ka.
Therefore, it has higher value of pKa.
(ii) The —COOH group in benzoic acid is attached to sp2 – carbon of the phenyl ring and is more acidic than acetic acid in which —COOH group is attached to sp3 – carbon atom of CH3 group. So, benzoic acid is stronger than acetic or acetic acid is weaker acid than benzoic acid.
Long Answer Questions
Question. Write the structures of the main products of the following reactions :
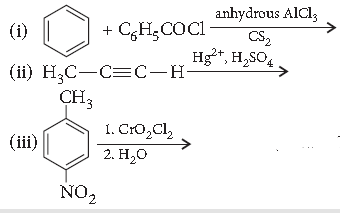
Answer.

Question. Write the products formed when CH3CHO
reacts with the following reagents :
(i) HCN
(ii) H2N — OH
(iii) CH3CHO in the presence of dilute NaOH
Answer.

Question. Write the products formed when ethanal reacts with the following reagents :
(i) CH3MgBr and then H3O+
(ii) Zn-Hg/conc. HCl
(iii) C6H5CHO in the presence of dilute NaOH
Answer.

Question. An organic compound (A) with molecular formula C8H8O forms an orange red precipitate with 2, 4-DNP reagent and gives yellow precipitate on heating with I2 and NaOH. It neither reduces Tollens’ reagent nor Fehling’s reagent, nor does it decolourise bromine water or Baeyer’s reagent. On drastic oxidation with chromic acid, it gives a carboxylic acid (B) having molecular formula C7H6O2. Identify the compounds (A) and (B) and explain the reactions involved.
Answer. (A) forms, 2, 4-DNP derivative. Therefore, it is an aldehyde or a ketone. Since it does not reduce Tollen’s or Fehling reagent, (A) must be a ketone. (A) responds to iodoform test. Compound (B), being an oxidation product of a ketone should be a carboxylic acid. The molecular formula of (B) indicates that it should be benzoic acid and compound (A) should, therefore, be a mono-substituted aromatic methyl ketone.

Question. An organic compound with molecular formula C5H10O does not reduce Tollens’ reagent but forms an addition compound with sodium hydrogen sulphite and gives a positive iodoform test. On vigorous oxidation, it gives ethanoic acid and propanoic acid. Identify the compound and write all chemical equations for the reactions.
Answer. The given compound does not reduce Tollens’ reagent, so it is not an aldehyde but the formation of addition compound with sodium hydrogen sulphite indicates it to be a carbonyl compound. Since this compound gives positive iodoform test, so it should

On oxidation, this compound gives ethanoic and propanoic acids which confirm its structure to be I.
Question. A ketone A(C4H8O), which undergoes a haloform reaction gives compound B on reduction. B on heating with sulphuric acid gives a compound C which forms monozonide D. D on hydrolysis in presence of zinc dust gives only acetaldehyde E. Identify A, B, C, D and E. Write the reactions involved.
Answer. The equations involved are :

Question. Identify A and E in the following series of reactions :

Answer.

Question. An organic compound (A) on treatment with ethyl alcohol gives a carboxylic acid (B) and compound (C). Hydrolysis of (C) under acidified conditions gives (B) and (D). Oxidation of (D) with KMnO4 also gives (B). (B) on heating with Ca(OH)2 gives (E) having molecular formula C3H6O. (E) does not give Tollen’s test and does not reduce Fehling’s solution but forms a 2, 4-dinitrophenylhydrazone. Identify (A), (B), (C), (D) and (E).
Answer.

E does not give Tollen’s reagent test and does not reduce Fehling’s solution as it is ketone

Question. An organic compound (A) on treatment with acetic acid in the presence of sulphuric acid produces an ester (B). (A) on mild oxidation gives (C). (C) with 50% KOH followed by acidification with dilute HCl generates (A) and (D). (D) with PCl5 followed by reaction with ammonia gives (E). (E) on dehydration produces hydrocyanic acid. Identify the compounds A, B, C, D and E.
Answer.

Therefore, (A) : Methyl alcohol
(B) : Methyl acetate
(C) : Formaldehyde
(D) : Formic acid
(E) : Formamide

