Please refer to MCQ Questions Chapter 11 The p-Block Elements Class 11 Chemistry with answers provided below. These multiple-choice questions have been developed based on the latest NCERT book for class 11 Chemistry issued for the current academic year. We have provided MCQ Questions for Class 11 Chemistry for all chapters on our website. Students should learn the objective based questions for Chapter 11 The p-Block Elements in Class 11 Chemistry provided below to get more marks in exams.
Chapter 11 The p-Block Elements MCQ Questions
Please refer to the following Chapter 11 The p-Block Elements MCQ Questions Class 11 Chemistry with solutions for all important topics in the chapter.
MCQ Questions Answers for Chapter 11 The p-Block Elements Class 11 Chemistry
Question. Name of structure of silicates in which three oxygen atoms of [SiO4]4- are shared is
(a) pyrosilicate
(b) sheet silicate
(c) linear chain silicate
(d) three dimensional silicate
Answer
B
Question. With respect to graphite and diamond, which of the statement(s) given is (are) correct ?
(a) Graphite is harder than diamond
(b) Graphite has higher electrical conductivity than diamond
(c) Graphite has higher thermal conductivity than diamond
(d) Graphite has higher C—C bond order than diamond
Answer
B,D
Question. Pb reacts with dilute HNO3 produces
(a) NO
(b) NH4NO3
(c) N2O5
Answer
A
Question. An oxide of an element is a gas and dissolves in water to give an acidic solution. The element belongs to
(a) II group
(b) IV group
(c) VIII group
(d) zero group
Answer
B
Question. Pyrosilicate ion is
(a) SiO22-
(b) SiO42-
(c) Si2O46–
(d) SiO3–
(e) Si2O76-
Answer
E
Question. Producer gas is the mixture of
(a) CO + N2
(b) CO+ H2
(c) CO + water vapour
(d) N2 + CH4
Answer
A
Question. The carbon based reduction method is not used for the extraction of
(a) tin from SnO2
(b) iron from Fe2O3
(c) aluminium from AI2O3
(d) magnesium from MgCO3 , CaCO3
Answer
C,D
Question. What product is formed on heating lead nitrate?
(a) PbO + NO+ O2
(b) PbO + NO2 + O2
(c) Pb + NO2
(d) PbO + N2
Answer
B
Question. Among the following substituted silanes, the one which will give rise to cross linked silicon polymer on hydrolysis is
(a) R4Si
(b) RSiCI3
(c) R2SiCI2
(d) R3SiCl
Answer
B
Question. Which one of the following elements reacts with steam ?
(a) C
(b) Ge
(c) Si
(d) Sn
Answer
D
Question. Dry ice is
(a) solid H2O
(c) solid N2O4
(b) solid CO2
(d) solid NH3
Answer
B
Question. Which type of silicate is shown in the given figure?

(a) Orthosilicate
(b) Pyrosilicate
(c) Metasilicate
(d) None of these
Andswer
D
Question. Identify B in the following reaction,
H4SiO4 →-H201000°C A →ΔCarbon B + CO
(a) corundum
(b) quartz
(c) silica
(d) carborundum
Answer
D
Question. The soldiers of Napolean army while at Alps during freezing winter suffered a serious problem as regards to the tin buttons of their uniforms. White metallic tin buttons got converted to grey powder. This transformation is related to
(a) a change in the crystalline structure of tin
(b) an interaction with nitrogen of the air at very low temperature
(c) a change in the partial pressure of oxygen in the air
(d) an interaction with water vapour contained in the humid air
Answer
A
Question. Which of the following does not contain silicon?
(a) Kaoline
(b) Agate
(c) Ruby
(d) Quartz
Answer
C
Question. Which of the following oxides is amphoteric in character?
(a) SnO2
(b) SiO2
(c) CO2
(d) CaO
Answer
A
Question. A metal, M forms chlorides in its + 2 and + 4 oxidation states. Which of the following statements about these chlorides is correct ?
(a) MCI2 is more volati le than MCl4
(b) MCI2 is more soluble in the anhydrous ethanol than MCI4
(c) MCI2 is more ionic than MCI4
(d) MCI2 is more easily hydrolysed than MCI4
Answer
C
Question. Which of the following is used for making optical instruments ?
(a) SiO2
(b) Si
(c) SiH4
(d) SiC
Answer
A
Question. In silica (SiO2), each silicon atom is bonded to
(a) two oxygen atoms
(b) four oxygen atoms
(c) one silicon and two oxygen atoms
(d) one silicon and four oxygen atoms
Answer
B
Question. Diamond is hard because
(a) all the four valence electrons are bonded to each carbon atom by covalent bonds
(b) it is a giant molecule
(c) it is made up of carbon atoms
(d) it cannot be burnt
Answer
A
Question. Buclaninster fullerene is
(a) pure graphite
(c) diamond
(b) C-60
(d) C-90
Answer
B
Question. A fibrous mineral which can withstand red hot flames without any damage is
(a) talc
(b) glass wool
(c) soap stone
(d) asbestos
Answer
D
Question. Which one of the following statements about the zeolites is false ?
(a) They are used as cation exchangers
(b) They have open structure which enables them to take up small molecules
(c) Zeolites are alurninosilicates having three dimensional network
(d) Some of the SiO44- units are replaced by AlO5–4 and AlO9-6 ions in zeolites
Answer
C
Question. White lead is
(a) PbCO3 · PbO
(b) PbCO3
(c) Pb(OH)2 · 2PbCO3
(d) PbSO4 · PbO
Answer
C
Question. Carborundum is
(a) SiC
(b) Al2O3 · H2O
(c) Al2(SO4)3
(d) AlCI3
Answer
A
Question. Silica is a/an
(a) acidic flux only
(b) gangue only
(c) basic flux only
(d) Both gangue and acidic flux
Answer
D
Question. What is the number of free electrons present on each carbon atom in graphite ?
(a) 0
(b) 3
(c) 2
(d) 1
Answer
D
Question. The stability of dihalides of Si, Ge, Sn and Pb increases steadily in the sequence
(a) GeX2 < SiX2 < SnX2 < PbX2
(b) SiX2 < GeX2 < PbX2 < SnX2
(c) SiX2 < GeX2 < SnX2 < PbX2
(d) PbX2 < SnX2 < GeX2 < SiX2
Answer
B
Question. Graphite is a soft solid lubricant extremely difficult to melt. The reason for this anomalous behaviour is that, graphite
(a) is a non-crystalline substance
(b) is an allotropic form of diamond
(c) bas molecules of variable molecular masses like polymers
(d) bas carbon atoms arranged in large plates of rings of strongly bound carbon atoms with weak interplate bonds
Answer
D
Question. The composition of the common glass is
(a) Na2O-CaO · 6SiO2
(b) Na2O· Al2O3 · SiO2
(c) CaO-Al2O3 -SiO2
(d) Na2O-CaO-6SiO2
Answer
A
Question. Which glass has the highest percentage of lead?
(a) Soda glass
(b) Flint glass
(c) Jena glass
(d) Pyrex glass
Answer
B
Question.
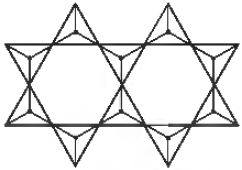
Silicate structure is unit of
(a)(Si4O11)n6n-
(b) (Si2o11)n2n-
(c) (Si2O3)
(d) (SiO4)-4
Answer
A
Question. Roasted tin stone ore after washing with water is known as
(a) block tin
(b) white tin
(c) black tin
(d) granulated tin
Answer
C
Question. The basic stmctural unit in silicates is
(a) SiO2
(b) [Si2O7]2-
(c) SiO.t tetrahedron
(d) [Si2O5]2-
Answer
C
Question. CO is poisonous gas, antidote for CO poisoning is
(a) carborundw.n
(b) carbogen
(c) carbonic acid
(d) pure oxygen
Answer
B
Question. In SiF62- and SiCl62- which one is known and why?
(a) SiF62- because of small size off
(b) SiF62- because of large size off
(c) SiCI62- because of small size of Cl
(d) SiCl62- because of large size of Cl
Answer
A
Question. One recently discovered allotrope of carbon (e.g. C60) is known as
(a) fluorine
(b) fullerene
(c) flourene
(d) freon
Answer
B
Question. Which of the following is not correct ?
(a) SiO2 is used as acidic flux
(b) The distance between the layers in graphite is 3.35x 10-3 cm
(c) SiO2 reacts with Na 2CO3 and liberates CO
(d) The hybridisation of C in graphite is sp2
Answer
C
Question. In the preparation of amorphous silicon, HF acid is used to remove
(a) Mg
(b) SiO2
(c) Si
(d) None of these
Answer
B
Question. In feldspar and zeolite, Si4+ ions are replaced by which ions?
(a) Oxide ion
(b) Hydroxide ion
(c) Aluminium ion
(d) Potassium ion
Answer
C
Question. Which gas is used in aerated water ?
(a) CO2
(b) SO2
(c) CO
(d) Water vapours
Answer
A
Question. Water gas is
(a) CO+ N2
(b) CO+ CO2 + CH4
(c) CO2 + N2
(d) CO+ H2
Answer
D
Question. On controlled hydrolysis and condensation, R3Si Cl yields
(a) R3Si—O—SiR3
(b) (—R3Si—O—SiR3—)n
(c) R3SiOH
R R
l l
(d) — Si — O — Si —
l l
O O
l l
— Si — O — Si —
l l
Answer
A
Question. Buckminster-fullerene is a variety of
(a) boron
(b) carbon
(c) ammonia
(d) fluorine
Answer
B
Question. Which element is used for making a transistor?
(a) Sn
(b) Sb
(c) Si
(d) Mg
Answer
C
Question. CO2 goes to air, causes green house effect and gets dissolved in water. What will be its effect on soil fertility and pH of the water?
(a) Increase
(b) Decrease
(c) Remain same
(d) None of these
Answer
D
Question. Solder is an alloy of lead with
(a) Cu
(b) Fe
(c) Sn
(d) Zn
Answer
C
Question. The tendency for catenation in group 14 elements varies in the order
(a) C » Si > Ge = Sn > Pb
(b) C « Si < Ge = Sn< Pb
(c) C » Si < Ge < Sn < Pb
(d) C » Si = Ge = Sn > Pb
Answer
A
Question. The purest form of coal is
(a) peat
(b) anthracite
(c) bituminous
(d) lignite
Answer
B
Question. Calorific value of producer gas is low because of
(a) high per cent of N2
(b) low per cent of CO2
(c) high per cent of CO
(d) low per cent of N2
Answer
A
Question. Crystalline form of silica is called
(a) crystalline silicon
(b) quartz
(c) rock
(d) talc
Answer
B
Question. Formula for agate is
(a) Na2SiO3
(b) K2O· SiO2 · Al2O3
(c) SiO2
(d) CaF2
Answer
C
Question. In silicon dioxide
(a) there are double bonds between silicon and oxygen atoms
(b) silicon atom is bonded to two oxygen atoms
(c) each silicon atom is surrounded by two oxygen atoms and each oxygen atom is bounded to two silicon atoms
(d) each silicon atom is surrounded by four oxygen atoms and each oxygen atom is bounded to two silicon atoms
Answer
D
Question. The thermal stability of CF4 is
(a) less than SiF4
(b) more than SiF4
(c) less than CCl4
(d) less than SiCl4
Answer
B
Question. Water glass is
(a) glass made of water
(b) sodium silicate
(c) calcium formate
(d) pyrex glass
Answer
B
Question. Which is likely to show inert-pair effect?
(a) K
(b) Mg
(c) Al
(d) Pb
Answer
D
Question. Addition of SnCI2 to HgCl2 gives precipitate
(a) white turning to red
(b) white turning to grey
(c) black turning to white
(d) None of these
Answer
B
Question. Carborundum is obtained when silica is heated at high temperature with
(a) carbon
(b) carbon monoxide
(c) carbon dioxide
(d) calcium carbonate
Answer
A
Question. The chemical formula of sindhur is
(a) PbO
(b) Pb3O4
(c) ZnO
(d) SnCl2
Answer
B
Question. Moderate electrical conductivity is shown by
(a) silica
(b) graphite
(c) diamond
(d) carborundum
Answer
B
Question. Quartz is an example of
(a) chain silicate
(b) sheet silicate
(c) cyclic s ilicate
(d) three dimensional network silicate
Answer
D
Question. When tin is treated with concentrated nitric acid
(a) it is converted into stannous nitrate
(b) it is converted into stannic nitrate
(c) it is converted into metastannic acid
(d) it becomes passive
Answer
C
Question. Which of the following is used in making printer’s ink, shoe polish, black varnish and paint ?
(a) Lamp black
(b) Bone black
(c) Carbon black
(d) None of these
Answer
A
Question. The incon-ect statement/s among the following is/are
I. NCl5 does not exist while PCl5 does.
II. Lead prefers to form tetravalent compounds.
III. The three C—O bonds are not equal in the carbonate ion.
IV. Both O+2 and NO are paramagnetic.
(a) I, III and IV
(b) I and IV
(c) II and III
(d) I and III
(e) IV Only
Answer
C

We hope you liked the above provided MCQ Questions Chapter 11 The p-Block Elements Class 11 Chemistry with solutions. If you have any questions please ask us in the comments box below.

