Please refer to MCQ Questions Chapter 13 Hydrocarbons Class 11 Chemistry with answers provided below. These multiple-choice questions have been developed based on the latest NCERT book for class 11 Chemistry issued for the current academic year. We have provided MCQ Questions for Class 11 Chemistry for all chapters on our website. Students should learn the objective based questions for Chapter 13 Hydrocarbons in Class 11 Chemistry provided below to get more marks in exams.
Chapter 13 Hydrocarbons MCQ Questions
Please refer to the following Chapter 13 Hydrocarbons MCQ Questions Class 11 Chemistry with solutions for all important topics in the chapter.
MCQ Questions Answers for Chapter 13 Hydrocarbons Class 11 Chemistry
Question. Among P , Q, Rand S, the aromatic compound (s) is/are
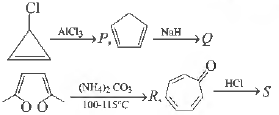
(a) P
(b) Q
(c) R
(d) S
Answer
C
Question. To get DDT chlorobenzene has to react will following compound in the presence of concentrated sulphuric acid
(a) trichloro ethane
(b) dichloro acetone
(c) dichloro acetaldehyde
(d) trichloro acetaldehyde
Answer
D
Question. Three fused benzene rings are found in
(a) naphthalene
(b) anthracene
(c) phenanthroline
(d) triphenyl methane
(e) None of the above
Answer
B
Question. In the oxidation of C6H5—CH2—CH3 by KMnO4 the product formed is
(a) C6H5—CH2—CHO
(b) C6H5—CH2—COOH
(c) C6H5—COOH
(d) C6H5—CH2—OH
Answer
D
Question. The electrophile in the nitration of benzene is
(a) No+2
(b) NO2
(c) NO+
(d) NO–2
Answer
A
Question. Which of the following molecules, in pure form is (are) unstable at room temperature ?
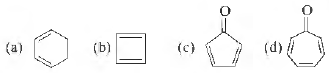
Answer
B
Question. The non-aromatic compound among the following is
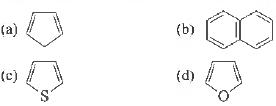
Answer
A
Question. Lindane can be obtained by the reaction of benzene with
(a) CH3Cl/ anhydrous AICl3
(b) C2H5I /anhydrous AICl3
(c) CH3COCI/ anhydrous AICl3
(d) Cl2 in sun light
Answer
D
Question. Which of the following molecules/species are aromatic
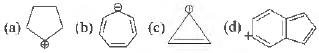
Answer
D
Question. Which will react with NaBH4 ?
(a) Benzoic acid
(b) Benzamide
(c) Cyclohexanone
(d) Acetic acid
Answer
C
Question. Suitable reagents A and B for the following reactions are
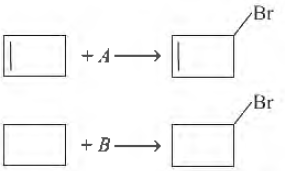
(a) Br, Br2
(b) Br2 , NBS
(c) NBS, NBS
(d) NBS,
Answer
D
Question.
On reductive ozonolysis yields
(a) 6-oxoheptanal
(b) 6-oxoheptanoic acid
(c) 6-hydroxyheptanaI
(d) 3-hydroxypentanal
Answer
A
Question. Cyclohexene on reaction with OsO4 followed by reaction with NaHSO3 gives
(a) cis-diol
(b) trans-diol
(c) epoxy
(d) alcohol
Answer
A
Question. What will be the octane number of best fuel?
(a) 80
(b) 81
(c) 74
(d) 65
Answer
B
Question. Petrol for aviation purpose must contain
(a) straight chain hydrocarbons
(b) aromatic hydrocarbons
(c) olefinic hydrocarbons
(d) highly branched chain paraffins
Answer
C
Question. TEL is a compound used as
(a) antibiotic
(b) antiseptic
(c) anti.knocking
(d) antioxidant
Answer
C
Question. Petrochemicals can be used to prepare
(a) synthetic fibres
(b) pesticides
(c) plastics
(d) All of these
Answer
B
Question. Gasoline with an octane number of 80 is equivalent in knocking characteristics to a mixture of heptane and iso-octane of the following composition
(a) 20% heptane + 80% iso-octane
(b) 90% heptane + 10% iso-octane
(c) 80% heptane + 20% iso-octane
(d) 10% heptane + 90% iso-octane
Answer
A
Question. Synthetic petrol is prepared by
(a) Wurtz reaction
(b) by distillation
(c) Fischer-Tropsch process
(d) None of the above
Answer
C
Question. Identify C in the following
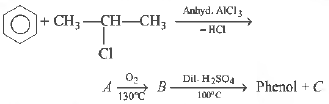
(a) ethanol
(b) propanone
(c) cumene hydroperoxide
(d) water
Answer
B
Question. What are the shapes of ethyne and methane?
(a) Square planar and linear
(b) Tetrahedral and trigonal planar
(c) Linear and tetrahedral
(d) Trigonal planar and linear
Answer
D
Question. Which of the following is a conjugated diene?
(a) CH3CH=C=CHCH3
(b) CH2= CHCH2CH= CH2
(c) CH2= CHCH2CH2CH= CH2
(d) CH2= C—CH= CH2ICH3
l
CH3
Answer
D
Question. Ozonolysis of an organic compound gives formaldehyde as one of the products. This confirms the presence of
(a) two ethylenic double bonds
(b) a vinyl group
(c) an iso-propyl group
(d) an acetylenic triple bond
Answer
B
Question. Ozonolysis of an organic compound A produces acetone and propionaldehyde in equimolar mixture. Identify A from the following compounds.
(a) 2 -methyl-1-pentene
(b) 1-pentene
(c) 2-pentene
(d) 2 -methyl-2-pentene
Answer
D
Question. In the following reaction, A and B, respectively are
A → C2H5Br → A
(a) C2 H4 , ale. KOH / Δ
(b) C2H5Cl,aq. KOH / Δ
(c) CH3O H,aq. KOH / Δ
(d) C2H2 , PBr3
Answer
A
Question. The conversion of propene to propanol is …. type of reaction.
(a) hydrogenation
(c) hydrolysis
(b) hydration
(d) dehydrogenation
Answer
B
Question. A gas decolourised by KMnO4 solution but gives no precipitate with ammoniacal cuprous chloride is
(a) ethane
(b) methane
(c) ethene
(d) acetylene
Answer
D
Question. Which reacts with ammoniacal AgNO3 ?
(a) Propyne
(b) 2-butyne
(c) 1, 3-butadiene
(d) Pentene
Answer
A
Question. Which is most aciclic of the following ?
(a) Methane
(b) Acetylene
(c) I -butene
(d) Neo-pentane
Answer
B
Question. The1mal decomposition of
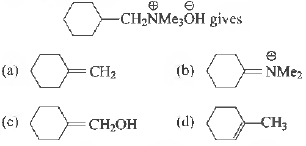
Answer
A
Question. An organic alkadiene on reductive ozonolysis produces
I. acetaldehyde
II. acetone
III. 2-methylpropane -1,3-dial The formula of alkadiene will be
(a) CH3C= CH CHCH = CHCH3
l l
CH3 CH3
(b) CH3CHCH = CCH = CHCH3
l l
CH3 CH3
(c) CH3C = CHCHC = CHCH3
l l
CH3 CH3
(d) CH3CH2CHCH = CHC= CH2
l l
CH3 CH3
Answer
A
Question. Oxidation of 1-butene with hot KMnO4 solution produces
(a) CH3CH2COOH + HCOOH
(b) CH3CH2COOH +CO2
(c) CH3COOH + CO2
(d) (CH3)2C=O + CO2
Answer
B
Question. An alkyne combines with a conjugated diene to give an unconjugated cycloalkadiene. The most likely title of this reaction is
(a) Schotten-Baumann reaction
(b) Hofrnann-bromamide reaction
(c) Pinacol-Pinacolone rearrangement
(d) Deils-Alder reaction
Answer
D
Question. A hydrocarbon of molecular formula C6H10 reacts with sodamide and the same on ozonolysis followed by hydrogen peroxide oxidation gives two molecules of carboxylic acids, one being optically active. Then, the hydrocarbon may be
(a) 1-hexyne
(b) 3-hexyne
(c) 3-methyl-1-pentyne
(d) 3, 3-dimethyl-1-butyne
Answer
D
Question. [A] →Lindlar’s catalyst CH3 — C=C — CH3 →Na in liq.NH3 [B]
[A] and [B] are respectively
(a) cis, trans-2-butene
(b) both trans-2-butene
(c) trans, cis-2-butene
(d) both cis-2-butene
Answer
A
Question. Acetylene does not react with
(a) Na
(b) ammoniacal AgNO3
(c) HCl
(d) NaOH
Answer
D
Question. Identify Z in the series,
HBr aq. KOH NaCO3
CH2 = CH2 → X → Y → Z
I2 excess
(a) C2H5I
(b) C2H5OH
(c) CHI3
(d) CH3CHO
Answer
D
Question. When 2-butyne is treated with Pd-BaSO4 , the product formed will be
(a) cis-2-butene
(b) trans-2-butene
(c) 1-butene
(d) 2-hydroxy butane
Answer
A
Question. Hydrogen bonding plays a central role in the following phenomena
(a) ice floats in water
(b) higher Lewis basicity of primary amines than tertiary amines in aqueous solutions
(c) formic acid is more acidic than acetic acid
(d) dimerisation of acetic acid in benzene
Answer
A,B.D
Question. 1-butyne on hydration gives
(a) butyne-1, 2-diol
(b) butane-1-ol
(c) butane-2-ol
(d) butane-2-one
Answer
D
Question. Which of the following reactions 2, 2-dibromopropane ?
(a) CH3—C= CH + 2HBr →
(b) CH3CH = CHBr + HBr →
(c) CH=CH + 2HBr →
(d) CH3 — CH= CH2 + HBr →
Answer
A
Question. In the sequence of reactions,
C2H4 →HBr X → AgCN y →H2/Ni[H] Z,
compound Z is
(a) N-rnethyl ethanamine
(c) N, N-clirnethylarnine
(b) N-propylamine
(d) ethyl cyanide
Answer
A
Question. A gas formed by the action of alcoholic KOH on ethyl iodide, decolourises alkaline KMnO4 . The gas is
(a) C2H6
(b) CH4
(c) C2H2
(d) C2H4
Answer
D
Question. The correct statement is
(a) cyclohexadiene and cyclohexene cannot be isolated with ease during controlled hydrogenation of benzene
(b) One mole each of benzene and hydrogen when reacted give 1/3 mole of cyclohexane and 2/3 mole 3 3 unreacted hydrogen
(c) Hydrogenation of benzene to cyclohexane is an endothermic process
(d) It is easier to hydrogenate benzene when compared to cyclohexene
Answer
A
Question. Which of the following will yield trans product from butyne?
(a) LiAlH4
(b) Na/Liq.NH3
(c) NaBH4
(d) Ni catalyst
Answer
B
Question. Which of the following is not aromatic
(a) 1, 3-cyclobutadiene
(b) pyridine
(c) furan
(d) thiophene
Answer
A
Question. n-C7H16 →V2O5, S00’C1020atm. A →CI2 B What is B in the above reaction?
(a) Benzyl chloride
(b) Benzal chloride
(c) Hexachlorobenzene
(d) Benzene hexachloride
Answer
B
Question. CH3 —C= C—CH3 →(ii) Zn/H2O(i) X
CH3—C—C—CH3 . In the above reaction X is
ll ll
O O
(a) HNO3
(b) O2
(c) O3
(d) KMnO4
Answer
D
Question. Benzene on treatment with a mixture of cone. HNO3 and cone. H2SO4 at 100°C gives
(a) nitrobenzene
(b) m-dinitrobenzene
(c) p-dinitrobenzene
(d) o-dinitrobenzene
Answer
B
Question. Reaction ofHBr with propene in the presence of peroxide gives
(a) iso-propyl bromide
(b) 3-bromo propane
(c) ally! bromide
(d) n-propyl bromide
Answer
D
Question. o-toluic acid on reaction with Br2 + Fe gives
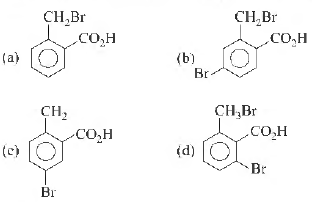
Answer
D
Question. Which one of these is not true for benzene?
(a) It fonns only one type of monosubstituted product
(b) There are three carbon-carbon single bonds and three carbon-carbon double bonds
(c) The heat of hydrogenation of benzene is less than the theoretical value
(d) The bond angle between the carbon-carbon bonds is
Answer
B
Question. The strongest ortho/para directing group is
(a) —NH2
(b) —CH3
(c) —Cl
(d) —C2H5
Answer
A
Question. Which of the following species is aromatic?
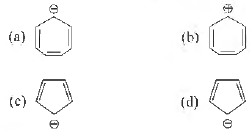
Answer
B
Question. Under which one of the following conditions, does the eaction, CH= CH + CH3OH →
CH3O—CH = CH2 take place?
(a) NH4OH / 80° C
(b) Cone. H2SO4 / 160° C
(c) Anhydrous ZnCl2 / 150° C
(d) Dilute HCl/fHF, 80°C
(e) CH3OK / 160- 200° C
Answer
E
Question. The major product Pin the following reaction is

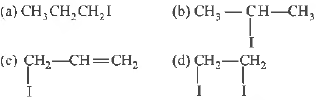
Answer
B
Question. In the following sequence of reactions, the alkene affords the compound B
CH3CH=CHCH3 →O3 A →ZnH2O B
The compound B is
(a) CH3CH2CHO
(c) CH3CH2COCH3
(b) CH3COCH3
(d) CH3CHO
Answer
D
Question. The hydrocarbon which can react with sodium in liquid ammonia is
(a) CH3CH2CH2C= CCH2CH2CH3
(b) CH3CH2C=CH
(c) CH3CH = CHCH3
(d) CH3CH2C = CCH2CH3
Answer
B
Question. Which one of the compound does not dissolve in concentrated H2SO4 ?
(a) Hexane
(b) Benzene
(c) Ethylene
(d) Aniline
Answer
A
Question. What is one of the products of the addition of HBr to 2-butene?
(a) 1-bromobutane
(b) 2-bromobutane
(c) 1,2-dibromobutane
(d) 2, 3-dibromobutane
Answer
B

We hope you liked the above provided MCQ Questions Chapter 13 Hydrocarbons Class 11 Chemistry with solutions. If you have any questions please ask us in the comments box below.

