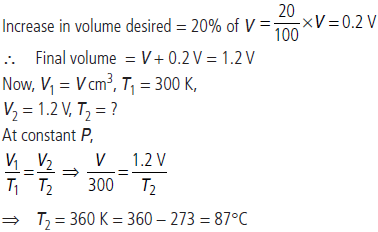Please refer to States of Matter Class 11 Chemistry Important Questions with solutions provided below. These questions and answers have been provided for Class 11 Chemistry based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these problem solutions as it will help them to gain more marks in examinations. We have provided Important Questions for Class 11 Chemistry for all chapters in your book. These Board exam questions have been designed by expert teachers of Standard 11.
Class 11 Chemistry Important Questions States of Matter
Very Short Answer Type Questions :
Question. Define the partial pressure of gas.
Answer : In a mixture of gases, the pressure exerted by the individual gas is called its partial pressure.
Question. Under which of the following two conditions applied together, a gas deviates most from the ideal behaviour?
Answer : At high pressure and low temperature, the gases deviate from ideal behaviour due to significant intermolecular forces and more than negligible size of the molecules.
Question. How is the partial pressure of a gas in a mixture related to the total pressure of the gaseous mixture?
Answer : Partial pressure of a gas
= Mole fraction of that gas × Total pressure
Question. Define compressibility factor.
Answer : The extent to which a real gas deviates from ideal behaviour can be conveniently studied in terms of quantity ‘Z’ called the compressibility factor, which is defined as Z = PV/nRT For an ideal gas, as PV = nRT, Z = 1
Question. The variation of pressure with volume of the gas at different temperatures can be graphically represented as shown in figure.
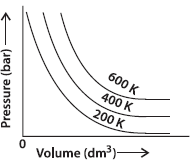
On the basis of this graph given, how will the volume of a gas change if its pressure is increased at constant temperature?
Answer : The volume of a gas decreases if the pressure on the gas is increased keeping the temperature constant.
Question. Name two intermolecular forces that exist between HF molecules in liquid state.
Answer : Dipole-dipole interactions and hydrogen bonding exist between HF molecules in liquid state.
Question. Why is boiling point of hydrogen fluoride higher than that of hydrogen chloride?
Answer : Boiling point of HF is higher than HCl due to extensive hydrogen bonding between H — F molecules.
H — F …… H — F …… H — F …… H — F
Question. Calculate the volume occupied by 4.0 mole of an ideal gas under NTP condition.
Answer : PV = nRT
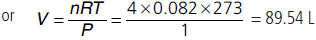
Question. Using the equation of state PV = nRT; show that at a given temperature, density of a gas is proportional to gas pressure P.
Answer :

Hence, density (d) of a gas ∝ P, because R, T and M are constants.
Question. Physical properties of ice, water and steam are very different. What is the chemical composition of water in all the three states.
Answer : The chemical composition of water remains same in all the physical states i.e., solid, liquid and gas.
Short Answer Type Questions :
Question. Calculate the total pressure (in bar) in a mixture of 8 g of dioxygen and 4 g of dihydrogen confined in a vessel of 1 dm3 at 27°C.
R = 0.083 bar dm3 K–1 mol–1
Answer : Partial pressure of oxygen gas,
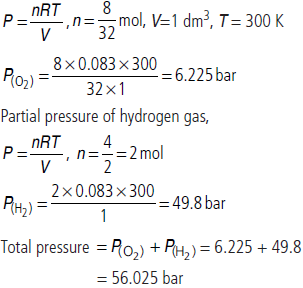
Question. Pressure versus volume graph for a real gas and an ideal gas are shown in the figure.
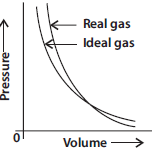
Answer the following questions on the basis of this graph.
(i) Interpret the behaviour of real gas with respect to ideal gas at low pressure.
(ii) Interpret the behaviour of real gas with respect to ideal gas at high pressure.
(iii) Mark the pressure and volume by drawing a line at the point where real gas behaves as an ideal gas.
Answer : (i) At low pressure real gas starts behaving like an ideal gas.
(ii) At high pressure gases deviate from ideal behaviour.

At point A real gas behaves as an ideal gas.
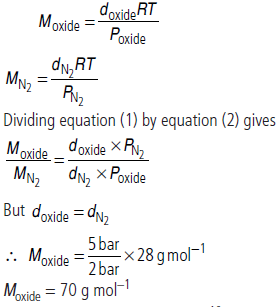
Question. At 0°C, the density of a certain oxide of a gas at 2 bar is same as that of dinitrogen at 5 bar. What is the molecular mass of the oxide?
Answer :
Question. Two moles of ammonia gas are enclosed in a vessel of 5 litre capacity at 27ºC. Calculate the pressure exerted by the gas, assuming that
(i) the gas behaves like an ideal gas (using ideal gas equation)
(ii) the gas behaves like a real gas (using van der Waals equation)
Given that for ammonia, a = 4.17 atm litre2 mol–2 and b = 0.037 litre mol–1.
Answer : Given, n = 2 moles, V = 5 litres, T = 27ºC = (27 + 273) K = 300 K
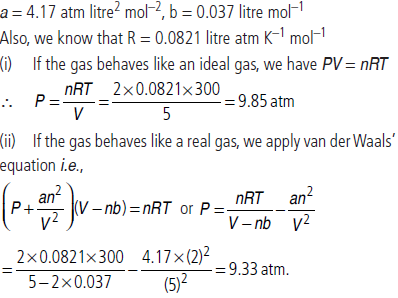
Question. 40 mL of O2 was collected at 100 °C and 1 bar pressure. Calculate its volume (in mL) at 273 K and 1.013 bar.
Answer :
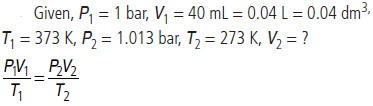
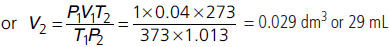
Question. Explain the difficulties faced by the mountaineers with respect to the air present around them. How is this difficulty solved?
Answer : At altitude, the atmospheric pressure is low. Hence, air is less dense. As a result, less oxygen is available for breathing. The person feels uneasiness, headache, etc. This is called altitude sickness. This difficulty is solved by carrying oxygen cylinders with them.
Question. 1 mole of sulphur dioxide occupies a volume of 350 mL at 27 °C and 5 × 106 Pa pressure. Calculate the compressibility factor of the gas. Is it less or more compressible than an ideal gas?
Answer : Compressibility factor, Z = PV/nRT

Thus, SO2 is more compressible than an ideal gas (which has Z = 1).
Question. 0.068 dm3 of a sample of nitrogen is collected over water at 20 °C and 0.92 bar. What will be the volume of dry nitrogen at STP (in mL) (Aqueous tension of water at 20 °C = 0.023 bar)?
Answer : Given P1 = 0.92 – 0.023 = 0.897 bar,
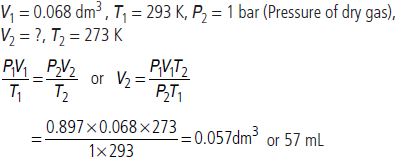
Question. An open beaker at 27°C is heated to 477°C. What percentage of air would have been expelled out?
Answer : Suppose the number of moles of gas present at 27°C in flask of volume V at pressure P is n1, then assuming ideal gas behaviour,
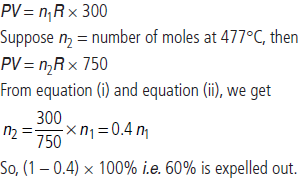
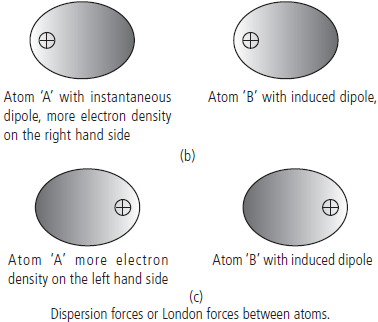
Question. Describe London forces or dispersion forces with example.
Answer : London or dispersion forces : This is the weakest intermolecular force. It is a temporary attractive force that results when the electrons in two adjacent atoms occupy positions that make the atoms to form temporary dipoles. This force is sometimes called an dipole-induced dipole attraction. Because of the constant motion of the electrons, an atom or molecule can develop a temporary (instantaneous) dipole when its electrons are distributed unsymmetrically about the nucleus.

Question. (a) 22 g of dry ice is placed in an evacuated bottle of 1 litre capacity and tightly stoppered. What would be the pressure inside the bottle in atm, when it is heated to 37°C?
(b) 3.12 g of sulphur is vapourised at 427°C and 760 mm pressure, when the vapours occupy a volume of 700 mL. Find the molecular formula of sulphur. (atomic mass of sulphur = 32).
Answer :
(a) W = 22 g CO2, V = 1 L, M = 44, T = 37 + 273 = 310 K, P = ?
Dry ice is solid CO2, which when heated in an evacuated bottle it is converted into gaseous CO2.
From ideal gas equation,
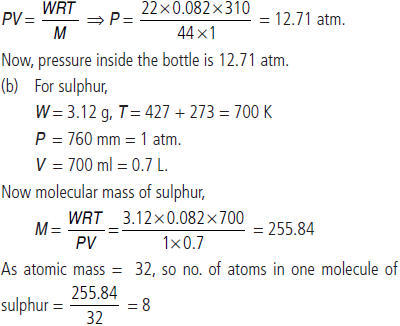
Hence, molecular formula of sulphur is S8.
Question. The drain cleaner, Drainex contains small bits of aluminium which react with caustic soda to produce dihydrogen. What volume of dihydrogen (in mL) at 20°C and one bar will be released when 0.15 g of aluminium reacts?
Answer : The reaction between aluminium and caustic soda is

Question. Calculate the volume (in m3) occupied by 2 moles of an ideal gas at 25 × 105 Nm–2 pressure and 300 K temperature.
Answer : According to ideal gas equation, PV = nRT
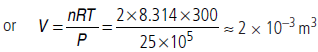
Question. 2.9 g of a gas at 95 °C occupied the same volume as 0.184 g of dihydrogen at 17 °C at the same pressure. What is the molar mass of the gas?
Answer : Case I : Let molar mass of gas be M g mol–1 Weight of gas = 2.9 g
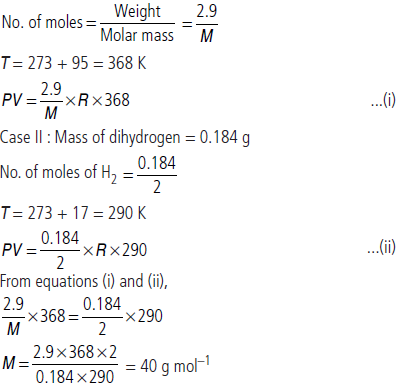
Question. Calculate the volume (in litres) occupied by 8.8 g of CO2 at 31.1°C and 1 bar pressure.
(R = 0.083 bar L K–1 mol–1)
Answer : According to ideal gas equation, PV = nRT

Question. What will be the pressure (in bar) of a gas mixture when 0.5 L of H2 at 0.8 bar and at 2.0 L of oxygen at 0.7 bar are introduced in a 1 L vessel at 27 °C?
Answer : Partial pressure of H2 :
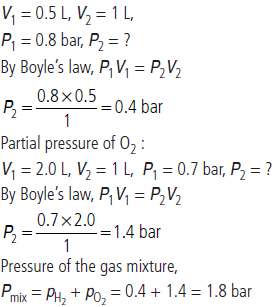
Question. On the basis of intemolecular forces and thermal energy explain why substances exist in three different states of matter?
Answer : The intermolecular forces tend to keep the molecules together but thermal energy tend to keep them apart. Thus, these two compete and the competition between these two (i.e., intermolecular forces and thermal energy) results in three states of matter.
(i) In a solid, the intermolecular forces predominate over the thermal energy and hence, the particles are held together in rigid, highly-oriented and close-packed structure.
(ii) In liquids, the intermolecular forces are no longer strong enough, however, these are still sufficient so that particles remain in each other’s environment, hence, liquids have sufficient mobility.
(iii) In gases, the thermal energy dominates the effect of intermolecular forces, thus, the gas molecules acquire the unrestricted and independent mobility in the vapour state.
Question. Explain the following :
(i) Boyle’s law
(ii) Avogadro’s law
Answer : (i) Boyle’s law : According to Boyle’s law, at constant temperature, the volume of a fixed amount of a gas is inversely proportional to its pressure, i.e., if volume increases, the pressure would decrease. This is because as volume increases, the number of molecules striking the walls of a container in a given time decreases leading to decrease in pressure.

Question. Which type of intermolecular forces exist among the following molecules?
(i) He atoms and HCl molecules
(ii) HF molecules
(iii) N2 molecules
(iv) HCl molecules
Answer : (i) Dipole-induced dipole forces
(ii) Hydrogen bonding
(iii) Dispersion forces
(iv) Dipole-dipole forces
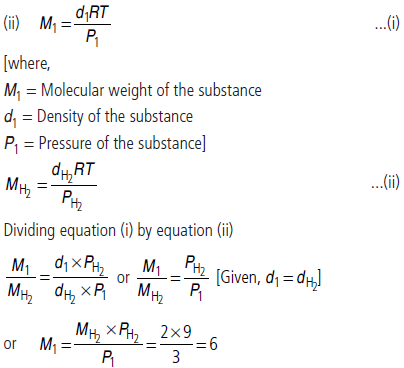
Question. (i) Out of CO2 and He, which gas have higher value of van der Waals’ constant ‘b’?
(ii) At 27°C density of a gaseous substance at 3 bar is same as that of hydrogen at 9 bar. What is molar mass of the substance?
Answer : (i) Since, CO2 molecules have larger size than that of He molecules, hence, CO2 has larger value of van der Waals’ constant ‘b’.
Question. On the basis of their interaction energy and thermal energy explain why
(i) a solid has high rigidity?
(ii) In gas, molecules are sufficiently apart from one another?
(iii) liquid has no definite shape?
Answer : (i) A solid has high rigidity because thermal motion is too weak to overcome the strong intermolecular forces of attraction.
(ii) In a gas, thermal energy is so high that the molecules cannot come close together. Hence, there are large empty spaces between them.
(iii) In a liquid, there is a reasonable balance between the attractive intermolecular forces and thermal energy. Hence, molecules in a liquid exist together, i.e., it is a condensed state of matter but there is no rigidity. That is why they have no definite shape.
Question. How much time (in years) would it take to distribute one Avogadro number of wheat grains, if 1010 grains are distributed each second?
Answer : Time taken to distribute 1010 grains = 1 sec. Time taken to distribute 6.023 × 1023 grains
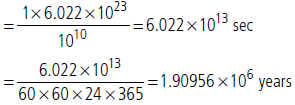
Question. (a) Which gas law is shown by the following graph?
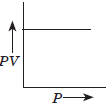
(b) At 25°C and 760 mm Hg pressure, a gas occupies 600 mL volume. What will be its pressure (in mmHg) at a height where temperature is 10°C and volume of the gas is 640 mL?
Answer : (a) Boyle’s law
(b) Applying gas equation (combined gas law),
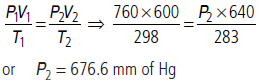
Question. Explain the physical significance of van der Waals’ parameters.
Answer : ‘a’ measures the intermolecular forces of attraction. The greater the value of ‘a’, the more will be intermolecular forces of attraction. ‘b’ measures volume occupied by molecules of a gas.
Question. A perfectly elastic spherical balloon of 0.2 m diameter was filled with hydrogen at sea level. What will be its diameter (in m) when it has risen to an altitude where the pressure is 0.65 atm? (Assume no change in temperature and atmospheric pressure at sea level).
Answer : If r1 is the radius of the balloon at sea level, then, volume

Pressure at the altitude (P2) = 0.65 atm (Given) As temperature remains constant, applying Boyle’s law,

Long Answer Type Questions :
Question. A liquefied petroleum gas (LPG) cylinder weighs 14.8 kg when empty. When full it weighs 29.0 kg and shows a pressure of 2.5 atm. In the course of use at 27 °C, the mass of the full cylinder is reduced to 23.2 kg. Find out the volume of the gas in cubic metres used up at the normal usage conditions and the final pressure inside the cylinder. Assume LPG to be n-butane with normal boiling point of 0 °C.
Answer : Weight of LPG originally present

Question. Answer the following :
(a) How are the van der Waals’ constants ‘a’ and ‘b’ related to the molecular size?
(b) Using van der Waals’ equation, calculate the constant ‘a’ when two moles of a gas confined in a 4 L flask exerts a pressure of 11.0 atmospheres at a temperature of 300 K. The value of ‘b’ is 0.05 L mol–1.
Answer : (a) The van der Waals’ constant ‘a’ is measure of intermolecular attractions. Therefore, the value of ‘a’ reflects the tendency of the gas to liquefy. The gas having larger value of ‘a’, will liquefy more easily. The van der Waals’ constant ‘b’ is a measure of the close-packed molecular volume. Thus the molecule of a gas having greater value of ‘b’ has bigger size.

Question. At temperature, T and pressure P, two ideal gases A and B are mixed. Show that the density d of the mixture is given by
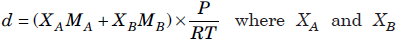
are the mole fractions and MA and MB are the molecular weights of the gases A and B respectively.
Answer : At temperature T and pressure P, two ideal gases, A and B are mixed.
nA and nB are the number of moles of A and B respectively. n is total moles of A and B present in the mixture.
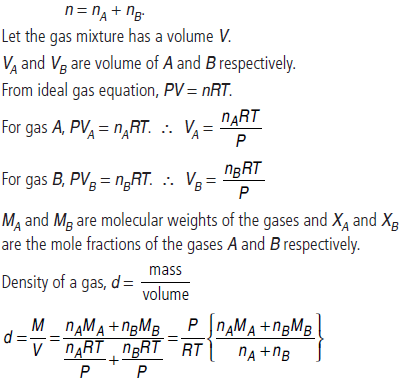

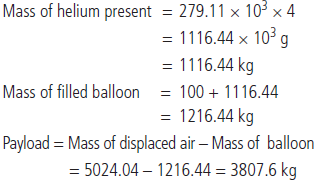
Question. Payload is defined as the difference between the mass of displaced air and the mass of the balloon. Calculate the payload (in kilograms) when a balloon of radius 10 m, mass 100 kg is filled with helium at 1.66 bar at 27°C. (Density of air =1.2 kg m–3 and R = 0.083 bar dm3 K–1 mol–1)
Answer :

Question. The volume of a gas is to be increased by 20% without changing the pressure. To what temperature (in °C) the gas must be heated if the initial temperature of the gas is 27 °C?
Answer : Suppose volume of gas at 27°C = V cm3