Please refer to The p-Block Elements Class 11 Chemistry Important Questions with solutions provided below. These questions and answers have been provided for Class 11 Chemistry based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these problem solutions as it will help them to gain more marks in examinations. We have provided Important Questions for Class 11 Chemistry for all chapters in your book. These Board exam questions have been designed by expert teachers of Standard 11.
Class 11 Chemistry Important Questions The p-Block Elements
Very Short Answer Type Questions :
Question. Describe the shapes of BF3 and BH4–. Assign the hybridisation of boron in these species.
Answer : BF3 has a planar triangular structure which arises from the sp2 hybrid orbitals.
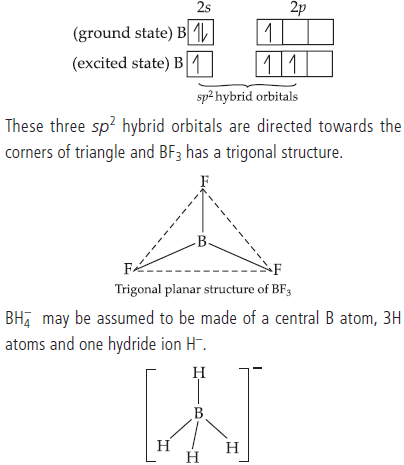
In order to accommodate the 3H atoms and one H– ion, B undergoes sp3 hybridisation yielding four orbitals, 3 of which contain one e– each and one is empty. The fourth, empty orbital accomodates the H– ion. Thus, the structure of BH4– is
tetrahedral.
Question. If B–Cl bond has a dipole moment, explain why BCl3 molecule has zero dipole moment.
Answer : The dipole moment of any molecule is the vector sum total of each of the dipole moments. In BCl3 molecule, although the B–Cl bonds individually are polar, the resultant dipole moment becomes zero.
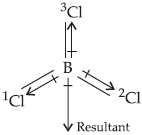
We can see that the dipole moments of B–1Cl and B–2Cl produce a resultant which is equal in magnitude but opposite in direction to B–3Cl and hence cancels it out. That is why the net dipole moment of BCl3 is zero.
Question. What is inert pair effect?
Answer : The tendency of s-electrons of the valence shell to participate in bond formation decreases down the group. This reluctance of the s-electrons to participate in bond formation is called inert pair effect.
Question. Suggest reasons why the B—F bond lengths in BF3 (130 pm) and BF–4 (143 pm) differ.
Answer : The bond length in any compound is dependent on the hybridisation of the central atom. Boron in BF3 is sp2 hybridised which means that the s-character is 33% and therefore, the bond length is shorter. Also due to similar size of both atoms and vacant p-orbital of B, a pp-pp back bonding from F to B occurs causes partial double bond character. This further decreases the bond length of B — F. In BF4–, the hybridisation of B is sp3 which means that the s-character is 25% and therefore, a longer bond length.
Question. Write reactions to justify amphoteric nature of aluminium.
Answer : Amphoteric substances are those that can react with both acids and bases. Aluminium reacts with HCl to liberate H2 gas as :

Question. How would you explain the lower atomic radius of Ga as compared to Al?
Answer : This can be understood from the variation in the inner core of the electronic configuration. The presence of additional 10 d-electrons offer only poor screening effect for the outer electron from the increased nuclear charge in gallium. Consequently, the atomic radius of gallium (135 pm) is less than that of aluminium (143 pm).
Question. Explain why is there a phenomenal decrease in ionization enthalpy from carbon to silicon?
Answer : Large decrease in ionisation potential from C to Si is due to increase in size of the atom and shielding effect.
Question. What are fullerenes? How are they prepared?
Answer : Fullerenes are the purest form of carbon, consisting of mainly C60 units. C60 unit has a shape of football, called Buckminsterfullerene. Fullerenes are prepared by heating graphite in an electric arc in the presence of inert gas such as helium or argon.
Question. Why does graphite act as a good lubricant?
Answer : Graphite has sheet like structure and it is slippery so, it can act as lubricant.
Question. What are electron deficient compounds? Are BCl3 and SiCl4 electron deficient species? Explain.
Answer : Electron deficient compounds are those where the central atom has less than 8 electrons in its outermost shell. Out of BCl3 and SiCl4, the former is an electron deficient compound since it has only 6 electrons in the outermost shell. SiCl4 is not an electron deficient compound.
However, it can accept electrons by expanding its octet due to presence of empty d-orbitals. Thus, it may form species like SiCl62–.
Short Answer Type Questions :
Question. Though fluorine is more electronegative than chlorine yet BF3 is a weaker Lewis acid than BCl3. Comment.
Answer : Due to back bonding in B — F, electron deficiency is compensated which makes it a weaker Lewis acid than BCl3. However, in B-Cl, back bonding is not significant due to much bigger size of 3p-orbital of Cl than vacant 2p-orbital of B.
Question. Describe the general trends in the following properties of the elements in Groups 13 and 14.
(i) Oxidation states
(ii) Atomic size
(iii) Nature of halide
Answer : (i) Oxidation states : For group 13 both +1 and +3 oxidation state are observed. +1 oxidation state becomes more stable as we move down the group due to inert pair effect. Boron does not show +3 oxidation state. For group 14 common oxidation state are +4 and +2. Tendency to show +2 oxidation state increases down the group.
(ii) Atomic size : Atomic radii of group13 elements increases down the group with exception Ga < Al due to presence of 10 d-electrons which offer poor screening effect. In group 14, there is a considerable increase in radius from C to Si, thereafter from Si → Pb a small increase is seen due to presence of completely filled d and f-orbitals.
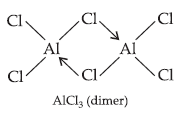
(iii) Nature of halides : Group 13 elements form trihalides (except TlI3). Due to electron deficient nature BCl3 accepts electrons and forms adducts. AlCl3 achieves stability by forming a dimer. Group 14 elements form halides
with formula MX2 and MX4. Except CCl4 other halides are easily hydrolysed. Stability of dihalides increases down the group.
Question. The +1 oxidation state in group 13 and +2 oxidation state in group 14 becomes more and more stable with increasing atomic number. Explain.
Answer : In group 13 and 14, as we move down the group, the tendency of s-electrons of the valence shell to participate in bond formation decreases. This is due to ineffective shielding of ns1 and ns2 electrons of the valence shell by intervening d- and f-electrons. This is called inert pair effect.
Due to this, ns1 and ns2 electrons of valence shell of group 13 and 14 are unable to participate in bonding. Hence, +1 and +2 oxidation states become more stable with increasing atomic number.
Question. Complete the following chemical equations and identify X, Y and Z.

Answer :
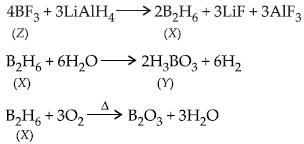
Question. Explain, why CO2 is a gas whereas SiO2 is a solid?
Answer : Silicon dioxide is a covalent three dimensional network solid due to absence of pp-pp bonding in SiO2 and very high Si — O bond enthalpy but in CO2 due to pπ-pπ bonding gives discrete molecules unlike SiO2. Thus, CO2 is a gas.
Question. What do you understand by
(a) inert pair effect
(b) allotropy and
(c) catenation?
Answer : (a) Inert pair effect : The reluctance of ns2 pair in p-block elements having higher atomic number to take part in bond formation is called inert pair effect.
(b) Allotropy : The existence of an element in more than one form having different physical properties but same or slightly different chemical properties is called allotropy.
(c) Catenation : The property by virtue of which a large number of atoms of the same element get linked together through covalent bonds resulting in the formation of long chains, branched chains and rings of different sizes is called catenation.
Question. How does AlCl3 act as a Lewis acid?
Answer : AlCl3 is a Lewis acid since it is an electron deficient halide. It has only six electrons in its outermost shell therefore, to complete its octet it accepts a lone pair of electrons and acts as a Lewis acid.
Question. Silicon forms SiF62– ion whereas corresponding fluoro compound of carbon is not known. Explain.
Answer : SiF62– ion exists because of presence of d-orbitals. Silicon expands its octet to give sp3d2 hybridisation and forms complexes or ions by accepting electron pairs from donor species like SiF62–. Carbon cannot exceed its covalency more than 4. Thus, CF62– is not known.
Question. Describe the general trends in the metallic character of the elements in groups 13 and 14.
Answer : Metallic character : Metallic character increases from boron to aluminium then decreases down the group for group 13 elements. Due to smaller size group 14 elements are less metallic. Metallic character increases gradually down the group. C (non-metal), Si,Ge (metalloid) Sn, Pb (metals).
Question. Explain the following :
(a) Electron gain enthalpy of chlorine is more negative as compared to fluorine.
(b) Pb4+ acts as an oxidising agent but Sn2+ acts as a reducing agent.
Answer : (a) Due to small size of fluorine there is inter-electronic repulsion which reduces its tendency to accepts electron.
(b) Pb4+ acts as oxidising agent because it has a tendency to exist in Pb2+ form which is more stable. Sn2+ is a reducing agent due to tendency to form Sn4+ compounds.
Question. Arrange the following in increasing order of the property indicated :
(a) SiCl2, GeCl2, SnCl2 and PbCl2 (stability)
(b) CO, SiO, SnO, GeO, PbO (basicity)
(c) SiF4, SiCl4, SiI4, SiBr4 (stability)
Answer : (a) The stability of dihalides increases down the group because divalent state becomes more and more stable as we move down the group.
SiCl2 < GeCl2 < SnCl2 < PbCl2
(b) Basicity of oxides increases down the group as metallic character increases.
CO < SiO < GeO < SnO < PbO
(c) Si—X bond strength decreases as the size of the halogen increases. The correct order is
SiI4 < SiBr4 < SiCl4 < SiF4
Question. Draw the structures of BCl3.NH3 and AlCl3 (dimer).
Answer :

Question. Explain why the following compounds behave as Lewis acids?
(i) BCl3
(ii) AlCl3
Answer : (i) BCl3 – Boron has 6 electrons in its outermost orbital and has a vacant p-orbital. Thus, it is an electron deficient compound hence acts as Lewis acid and accepts a lone pair of electrons.
(ii) AlCl3 is also an electron deficient compound and acts as Lewis acid. It generally forms a dimer to achieve stability.
Question. Carbon and silicon both belong to the group 14, but inspite of the stoichiometric similarity, the dioxides, (i.e., carbon dioxide and silicon dioxide), differ in their structures. Comment.
Answer : Due to absence of d-orbitals multiple pp-pp bonding is present in carbon dioxide hence CO2 is linear (O C O) with sp hybridisation. SiO2 has discrete single bonded structure in a tetrahedral manner.

Question. Explain the following :
(i) Boron does not exist as B3+ ion.
(ii) Discuss the Lewis acid nature of boron halides.
Answer : (i) Due to small size of boron, the sum of its first three ionisation enthalpies is very high, hence, it does not exist in +3 form.
(ii) The Lewis acid character of boron trihalides follows the order :
BI3 > BBr3 > BCl3 > BF3.
The above order is just the reverse of the expected order on the basis of relative electronegativities of the halogens. This can be explained on the basis of the tendency of the halogen atom to back-donate its electrons to the boron atoms resulting in the formation of an additional pπ–pπ bond. This type of bond formation is known as dative or back bonding.
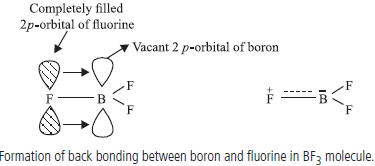
As a result of back donation of electrons from fluorine to boron, the electron deficiency of boron atom gets compensated and therefore, the Lewis acid character of BF3 decreases. The tendency to form pp–pp bond is maximum in the case of BF3 and falls rapidly as we move to BCl3 and BBr3
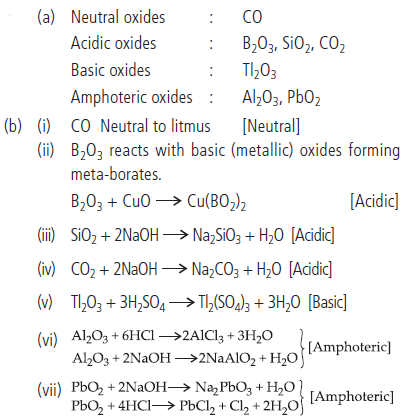
Question. (a) Classify following oxides as neutral, acidic, basic or amphoteric:
CO, B2O3, SiO2, CO2, Al2O3, PbO2, Tl2O3
(b) Write suitable chemical equations to show their nature.
Answer :
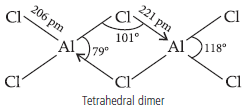
Question. BCl3 exists as monomer whereas AlCl3 is dimerised through halogen bridging. Give reason. Explain the structure of the dimer of AlCl3 also.
Answer : Due to absence of d-orbitals in boron, it exists as an electron deficient monomer and achieves stability through accepting electrons from a base like NH3. It cannot exists as dimer due to small size of B which cannot accomodate bigger size 4 Cl atoms around it. AlCl3 achieves stability by forming a dimer.
Question. What are allotropes? Sketch the structure of two allotropes of carbon namely diamond and graphite. What is the impact of structure on physical properties of two allotropes?
Answer : The property due to which an element exists in two or more forms which differ in their physical and some of the chemical properties is known as allotropy and the various forms are called allotropes or allotropic modifications. Carbon exists in two allotropic forms crystalline and amorphous. The crystalline forms are diamond and graphite.

Diamond due to extended covalent bonding is the hardest natural substance on the earth. Graphite has layer of
sheets which are held by weak van der Waals’ forces thus, it can be cleaved easily between layers which makes it soft and slippery.
Question. Give reason why CCl4 is immiscible in water, whereas SiCl4 is easily hydrolysed.
Answer : CCl4 cannot be hydrolysed by water because carbon atom can not accommodate lone pair of electrons from oxygen atom of water due to absence of d-orbital. SiCl4 can be hydrolysed to give Si(OH)4 due to presence of d-orbitals.

Question. Explain the difference in properties of diamond and graphite on the basis of their structures.
Answer :


Long Answer Type Questions :
Question. Three pairs of compounds are given below. Identify that compound in each of the pairs which has group 13 element in more stable oxidation state. Give reason for your choice. State the nature of bonding also.
(i) TlCl3 , TlCl
(ii) AlCl3 , AlCl
(iii) InCl3 , InCl
Answer : (i) TlCl3, TlCl – TlCl is in more stable oxidation state (+1 O.S. more stable). It is ionic in nature.
(ii) AlCl3,AlCl – AlCl3 is more stable (+3 oxidation state). It is covalent in nature.
(iii) InCl3, InCl – InCl3 is relatively more stable than InCl due to higher stability of +3 oxidation state. It is covalent in nature.
Question. Explain the following :
(a) Carbon shows catenation property but lead does not.
(b) Lead does not form PbI4.
(c) Pb4+ acts as an oxidising agent but Sn2+ acts as a reducing agent.
Answer : (a) Property of catenation is maximum in carbon because C — C bonds are very strong due to smaller size. The tendency of catenation decreases down the group due to increase in size and decrease in electronegativity.
(b) Pb + 2I2 → PbI4
I– is a good reducing agent and therefore, reduces Pb (IV) to Pb (II) easily. That is why, PbI4 does not exist.
(c) Pb4+ acts as an oxidising agent because it has a tendency to exist in Pb2+ form which is more stable. Sn2+ is a reducing agent due to tendency to form Sn4+ compounds.
Question. Describe the general trends in the following properties of the elements of groups 13.
(i) Atomic size
(ii) Ionisation enthalpy
(iii) Metallic character
(iv) Oxidation states
(v) Nature of halides.
Answer : (i) Atomic size : Atomic radii of group 13 elements increase down the group with the exception that atomic radius of Ga is less than that of Al due to the presence of 10 d-electrons which offer poor screening effect for the outer electrons from the increased nuclear charge in Ga.
(ii) Ionisation enthalpy : For group 13 elements, the trend of ionisation enthalpy is B > Al < Ga > In < Tl. This is due to increase in size and low screening effect of d- and f- electrons.
(iii) Metallic character : Metallic character increases from boron to aluminium then decreases down the group.
(iv) Oxidation states : For group 13 elements, both +1 and +3 oxidation states are observed. The +1 oxidation state becomes more stable as we move down the group due to inert pair effect. Boron does not show +3 oxidation state.
(v) Nature of halides : Group 13 elements form trihalides (except Tll3). Due to electron deficient nature, BCl3 accepts electrons and forms adducts. AlCl3 achieves stability by forming a dimer.
Question. (a) Boron fluoride exists as BF3 but boron hydride doesn’t exist as BH3. Give reason. In which form does it exist? Explain its structure.
(b) A tetravalent element forms monoxide and dioxide with oxygen. When air is passed over heated element (1273 K), producer gas is obtained. Monoxide of the element is a powerful reducing agent and reduces ferric oxide to iron. Identify the element and write formulas of its monoxide and dioxide. Write chemical equations for the formation of producer gas and reduction of ferric oxide with the monoxide.
Answer : (a) Due to non-availability of d-orbitals, boron is unable to expand its octet hence, it exists as BF3 and is electron deficient compound. Due to back bonding, electron deficiency of BF3 is compensated. But in boron hydride, hydrogen atoms does not have lone pairs for back bonding thus, to compensate electron deficiency it exists in the form of diborane.
In the structure of diborane, four terminal hydrogen atoms and two boron atoms are in one plane. Above and below this plane, there are two bridging hydrogen atoms. The four terminal B — H bonds are regular two centre two electron bonds while the two bridge B — H — B bonds are different and are three centre – two electron bonds.

Question. Explain the following:
(i) Why PbO2 is a stronger oxidising agent than SnO2?
(ii) Why ionisation enthalpy of Ga is higher than that of Al?
(iii) Thallous compounds (Tl+) are more stable than thallic (Tl3+) compounds. Why?
Answer : (i) Lead compounds in +2 oxidation state are more stable than +4 oxidation state hence are stronger oxidising agents. Due to stronger inert pair effect Pb2+ is more stable than Sn2+.
(ii) As we move from Al to Ga, due to poor shielding of the nucleus by 3d-electrons, the effective nuclear charge acting on Ga is slightly higher than that on Al. As a result, ionisation enthalpy of Ga is higher than that of Al.