Please refer to the Redox Reactions Revision Notes given below. These revision notes have been designed as per the latest NCERT, CBSE and KVS books issued for the current academic year. Students will be able to understand the entire chapter in your class 11th Chemistry book. We have provided chapter wise Notes for Class 11 Chemistry as per the latest examination pattern.
Revision Notes Chapter 8 Redox Reactions
Students of Class 11 Chemistry will be able to revise the entire chapter and also learn all important concepts based on the topic wise notes given below. Our best teachers for Grade 11 have prepared these to help you get better marks in upcoming examinations. These revision notes cover all important topics given in this chapter.
Oxidation number denotes the oxidation state of an element in a compound ascertained according to a set of rules formulated on the basis that electron in a covalent bond belong sentirely to more electronegative element.
Calculation of oxidation number:
1. O. S. of all the elements in their elemental form (in standard state) is taken as zero O. S. of elements in Cl2, F2, O2, P4, O3, Fe(s), H2, N2, C(graphite) is zero.
2. Common O. S. of elements of group one (1st) is one. Common O. S. of elements of group two (2nd) is two.
3. For ions composed of only one atom, the oxidation number is equal to the charge on the ion.
4. The oxidation number of oxygen in most compounds is –2.While in peroxides (e.g., H2O2, Na2O2), each oxygen atom is assigned an oxidation number of –1, in superoxides (e.g., KO2,RbO2) each oxygen atom is assigned anoxidation number of –(½).
5. In oxygen difluoride (OF2) and dioxygen difluoride (O2F2), the oxygen is assignedan oxidation number of +2 and +1,respectively.
6. The oxidation number of hydrogen is +1 but in metal hydride its oxidation no. is–1.
7. In all its compounds, fluorine has anoxidation number of –1.
8. The algebraic sum of the oxidation number of all the atoms in a compound must be zero.
9. In polyatomic ion, the algebraic sum of all the oxidation numbers of atoms of the ion must equal the charge on the ion.
Stock notation:the oxidation number is expressed by putting a Romannumeral representing the oxidation number in parenthesis after the symbol of the metal inthe molecular formula. Thus aurous chloride and auric chloride are written as Au(I)Cl and Au(III)Cl3. Similarly, stannous chloride and stannic chloride are written as Sn(II )Cl2and Sn(IV)Cl4.
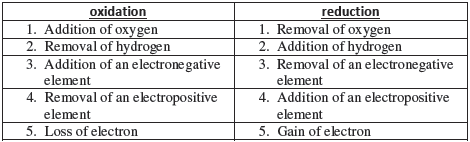
Oxidation: An increase in the oxidation number
Reduction: A decrease in the oxidation number
Oxidising agent: A reagent which can increase the oxidation number of an element in a given substance. These reagents are called as oxidants also.
Reducing agent: A reagent which lowers the oxidation number of an element in a given substance. These reagents are also called as reductants.
Redox reactions: Reactions which involve change in oxidation number of the interacting species
Balancing of redox reactions:
Oxidation Number Method:
Write the net ionic equation for the reaction of potassium dichromate(VI), K2Cr2O7 with sodium sulphite,Na2SO3, in an acid solution to give chromium(III) ion and the sulphate ion.
Step 1: The skeletal ionic equation is:
Cr2O7 2–(aq) + SO32–(aq) → Cr3+(aq)+ SO42–(aq)
Step 2: Assign oxidation numbers forCr and S
+6 –2 +4 –2 +3 +6 –2
Cr2O7 2–(aq) + SO32–(aq) → Cr3+(aq)+ SO42–(aq)
Step 3: Calculate the increase anddecrease of oxidation number, and make them equal:
+6 –2 +4 –2 +3 +6
Cr2O7 2–(aq) + 3SO32–(aq) → 2Cr3+(aq)+ 3SO42–(aq)
Step 4: Balance the charge by adding H+as the reaction occurs in the acidic medium,
Cr2O7 2–(aq) + 3SO32–(aq) 8H+→ 2Cr3+(aq)+ 3SO42–(aq)
Step 4: For reactions occurring in acidicmedium, add H2O to balance O atoms and H+to balance H atoms.
Cr2O7 2– (aq) +14 H+→ Cr3+(aq) + 7H2O (l)
Step 5: Add electrons to one side of the half reaction to balance the charges. If need be,make the number of electrons equal in the two half reactions by multiplying one or both half reactions by appropriate coefficients.
Fe2+(aq) → Fe3+ (aq) + e–
Cr2O7 2– (aq) + 14H+ (aq) + 6e– → 2Cr3+(aq) +7H2O (l)
6Fe2+ (aq) →6 Fe3+(aq) +6 e–
Step 6: We add the two half reactions to achieve the overall reaction and cancel the electrons on each side. This gives the net ionic equation as :
6Fe2+ (aq)+ Cr2O7 2– (aq) + 14H+ (aq) → 6 Fe3+(aq) +2Cr3+(aq) + 7H2O(l)
redox couple: A redox couple is defined as having together the oxidised and reduced forms of a substance taking part in an oxidation or reduction half reaction.
Represented as Zn2+/Zn and Cu2+/Cu.
Electrochemical cells: Electrochemical cells are the devices which are used to get electric current by sing chemical reaction

Electrode potential: The potential associated with each electrode is known as electrode potential. If the concentration of each species taking part in the electrode reaction is unity (if any gas appears in the electrode reaction, it is confined to 1 atmospheric pressure) and further there action is carried out at 298K, then the potential of each electrode is said to be the Standard Electrode Potential.
• SHE is used to measure electrode potential and its standard electrode potential is taken as 0.00 V.



