VBQs The p – Block Elements Class 12 Chemistry with The p – Block Elements has been provided below for standard students. We have provided chapter wise VBQ for Class 12 Chemistry with The p – Block Elements. The following The p – Block Elements Class 12 Chemistry value based questions with answers will come in your exams. Students should understand the concepts and learn the solved cased based VBQs provided below. This will help you to get better marks in class 12 examinations.
The p – Block Elements VBQs Class 12 Chemistry
Question. Noble gases do not react with other elements because
(a) they have completely filled valence shell (ns2np6)
(b) the sizes of their atoms are very small
(c) they are not found in abundance
(d) they are monoatomic
Answer
A
Question.The last member of the family of inert gases is
(a) argon
(b) radon
(c) xenon
(d) neon
Answer
B
Question. The correct order of heat of formation of halogen acids is
(a) HI > HBr > HCl > HF
(b) HF > HCl > HBr > HI
(c) HCl > HF > HBr > HI
(d) HCl > HBr > HF > HI
Answer
B
Question. Which of the following noble gases has the highest positive electron gain enthalpy value?
(a) Helium
(b) Krypton
(c) Argon
(d) Neon
Answer
D
Question. Number of unpaired electrons in inert gas is
(a) zero
(b) 8
(c) 4
(d) 18
Answer
A
Question. Which of the following is most volatile ?
(a) HI
(b) HBr
(c) HCl
(d) HF
Answer
C
Question. Gradual addition of electronic shells in the noble gases causes a decrease in their
(a) ionisation energy
(b) atomic radius
(c) boiling point
(d) density
Answer
A
Question. The most abundant inert gas in the atmosphere is
(a) He
(b) Ne
(c) Ar
(d) Kr
Answer
C
Question. Fluorine is a stronger oxidising agent than chlorine in aqueous solution. This is attributed to many factors except
(a) heat of dissociation
(b) ionisation potential
(c) heat of hydration
(d) electron affinity
Answer
B
Question. Which of the noble gas has highest polarisability?
(a) He
(b) Ar
(c) Kr
(d) Xe
Answer
D
Question. The noble gas which was discovered first in the sun and then on the earth
(a) argon
(b) xenon
(c) neon
(d) helium
Answer
D
Question. Interhalogen compounds are more reactive than the individual halogen because
(a) two halogens are present in place of one
(b) they are more ionic
(c) their bond energy is less than the bond energy of the halogen molecule
(d) they carry more energy
Answer
C
Question. Which inert gas show abnormal behaviour on liquefaction
(a) Xe
(b) He
(c) Ar
(d) Kr
Answer
B
Question. The ease of liquefaction of noble gases increases in the order
(a) He < Ne < Ar < Kr < Xe
(b) Xe < Kr < Ne < Ar < He
(c) Kr < Xe < He < Ne < Ar
(d) Ar < Kr < Xe < Ne < He
Answer
A
Question. Among F, Cl, Br and I the lowest ionization potential will be of
(a) fluorine
(b) chlorine
(c) bromine
(d) iodine
Answer
D
Question. In which of the following reactions chlorine is both reduced and oxidized?
(a) 2KMnO4 + 16HCl → 2KCl + 2MnCl2 + 8H2O + 5Cl2
(b) 6NaOH + 3Cl2 → 5NaCl + NaClO3 + 3H2O
(c) NH3 + 3Cl2 → NCl3 + 3HCl
(d) I2 + 6H2O + 5Cl2 → 2HIO3 + 10HCl
Answer
B
Question. In XeF2, XeF4, XeF6 the number of lone pairs on Xe are respectively
(a) 2, 3, 1
(b) 1, 2, 3
(c) 4, 1, 2
(d) 3, 2, 1.
Answer
D
Question. Gaseous HCl is a poor conductor of electricity while its aqueous solution is a good conductor this is because
(a) H2O is a good conductor of electricity
(b) a gas cannot conduct electricity but a liquid can
(c) HCl gas does not obey Ohm’s law, whereas the solution does
(d) HCl ionises in aqueous solution
Answer
D
Question. Total number of lone pair of electrons in XeOF4 is
(a) 0
(b) 1
(c) 2
(d) 3
Answer
B
Question. Which of the following are peroxoacids of sulphur?
(a) H2SO5 and H2S2O8
(b) H2SO5 and H2S2O7
(c) H2S2O7 and H2S2O8
(d) H2S2O6 and H2S2O7
Answer
A
Question. Which of the following is not the characteristic of interhalogen compounds ?
(a) They are more reactive than halogens
(b) They are quite unstable but none of them is explosive
(c) They are covalent in nature
(d) They have low boiling points and are highly volatile.
Answer
D
Question. Which one of the following reactions of xenon compounds is not feasible?
(a) 3XeF4 + 6H2O → 2Xe + XeO3 +12HF +1.5O2
(b) 2XeF2 + 2H2O → 2Xe + 4HF + O2
(c) XeF6 +RbF → Rb[XeF7]
(d) XeO3 + 6HF → XeF6 + 3H2O
Answer
D
Question. Noble gases are group of elements which exhibit very
(a) high chemical activity
(b) low chemical activity
(c) minimum electronegativity
(d) much paramagnetic properties
Answer
B
Question. Which of the following halogens exhibit only one oxidation state in its compounds ?
(a) Bromine
(b) Chlorine
(c) Fluorine
(d) Iodine
Answer
C
Question. Which element out of He, Ar, Kr and Xe forms least number of compounds ?
(a) He
(b) Ar
(c) Kr
(d) Xe
Answer
A
Question. Which one of the following noble gases is not found in the atmosphere
(a) Rn
(b) Kr
(c) Ne
(d) Ar
Answer
A
Question. XeF4 involves which hybridization
(a) sp
(b) sp2
(c) sp2d
(d) sp3d2
Answer
D
Question. A radioactive element X decays to give two inert gases X is
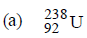

(c) Both (a) and (b)
(d) Neither (a) nor (b)
Answer
B
Question. Shape of XeOF4 is
(a) octahedral
(b) square pyramidal
(c) pyramidal
(d) T-shaped
Answer
B
Question. On addition of conc. H2SO4 to a chloride salt, colourless fumes are evolved but in case of iodide salt, violet fumes come out. This is because
(a) H2SO4reduces HI to I2
(b) HI is of violet colour
(c) HI gets oxidised to I2
(d) HI changes to HIO3
Answer
C
Question. Which has trigonal bipyramidal shape ?
(a) XeOF4
(b) XeO3
(c) XeO3F2
(d) XeOF2
Answer
B
Question. The number of lone pair of electrons present on Xe in XeF2 is
(a) 3
(b) 4
(c) 2
(d) 1
Answer
A
Question. Hybridization and structure of XeF4 is
(a) sp3d, trigonal bipyramidal
(b) sp3, tetrahedral
(c) sp3d2, square planar
(d) sp3d2, hexagonal
Answer
C
Question. Number of lone pairs of electrons on Xe atoms XeF2, XeF4 and XeF6 molecules are respectively
(a) 3, 2 and 1
(b) 4, 3 and 2
(c) 2, 3 and 1
(d) 3, 2 and 0
Answer
A
Question. Which one of the following is correct pair with respect to molecular formula of xenon compound and hybridization state of Xenon in it?
(a) XeF4, sp3
(b) XeF2, sp
(c) XeF2, sp3d
(d) XeF4, sp2
Answer
C
Question. Which is a planar molecule ?
(a) XeO4
(b) XeF4
(c) XeOF4
(d) XeO2F2e
Answer
B
Question. Which statement about noble gases is not correct?
(a) Xe forms XeF6
(b) Ar is used in electric bulbs
(c) Kr is obtained during radioactive disintegration
(d) He has the lowest b.pt among all the noble gases
Answer
C
Question. The geometry of XeF6 is
(a) planar hexagon
(b) regular octahedron
(c) distorted octahedron
(d) square bipyramid
Answer
C
Question. Which of the following statements are true?
(i) Only type of interactions between particles of noble gases are due to weak dispersion forces.
(ii) Ionisation enthalpy of molecular oxygen is very close to that of xenon.
(iii) Hydrolysis of XeF6 is redox reaction.
(iv) Xenon fluorides are not reactive.
(a) (i) and (iii)
(b) (i) and (ii)
(c) (ii) and (iii)
(d) (iii) and (iv)
Answer
B
Question. Trigonal bipyramidal geometry is shown by :
(a) XeO3F2
(b) XeO3F2
(c) FXeOSO2F
(d) [XeF8]2–
Answer
B
Question. Sea divers go deep in the sea water with a mixture of which of the following gases
(a) O2 and He
(b) O2 and Ar
(c) O2 and CO2
(d) CO2 and Ar
Answer
A
Question. Argon is used
(a) in filling airships
(b) to obtain low temperature
(c) in high temperature welding
(d) in radiotherapy for treatment of cancer
Answer
C
Question. Which of the following has sp3 hybridization ?
(a) XeO3
(b) BCl3
(c) XeF4
(d) BBr3
Answer
A
Question. Which of the following is the life saving mixture for an asthma patient ?
(a) Mixture of helium and oxygen
(b) Mixture of neon and oxygen
(c) Mixture of xenon and nitrogen
(d) Mixture of argon and oxygen
Answer
A
Question. Which of the following is used to produce and sustain powerful superconducting magnets to form an essential part of NMR spectrometer ?
(a) Ar
(b) Ne
(c) Rn
(d) He
Answer
D
Question. The coloured discharge tubes for advertisement mainly contain
(a) xenon
(b) helium
(c) neon
(d) argon
Answer
C
Question. Noble gases are used in discharge tubes to gives different colours. Reddish orange glow is due to
(a) Ar
(b) Ne
(c) Xe
(d) Kr
Answer
B
Question. Which of the following element has the property of diffusing through most commonly used laboratory materials such as rubber, glass or plastics.
(a) Xe
(b) Rn
(c) He
(d) Ar
Answer
C
Question. Which one of the following statements regarding helium is incorrect ?
(a) It is used to produce and sustain powerful superconducting magnets.
(b) It is used as a cryogenic agent for carrying out experiments at low temperatures.
(c) It is used to fill gas balloons instead of hydrogen because it is lighter and non-inflammable.
(d) It is used in gas-cooled nuclear reactors.
Answer
C
MATCHING TYPE QUESTIONS
Question. Match the columns.
Column-I Column-II
(A) SF4 (p) Tetrahedral
(B) BrF3 (q) Pyramidal
(C) BrO3– (r) Sea-saw shaped
(D) NH4+ (s) Bent T-shaped
(a) A – (r), B – (q), C – (p), D – (s)
(b) A – (r), B – (s), C – (q), D – (p)
(c) A – (p), B – (q), C – (r), D – (s)
(d) A – (p), B – (s), C – (r), D – (q)
Answer
B
Question. Match the columns
Column – I Column – II
(A) Used in manufacture (p) Ammonia
of calcium cyanamide
(B) Used in manufacture (q) Nitric acid
of nitric acid
(C) Used in pickling of (r) Dinitrogen
stainless steel
(a) A – (r), B – (p), C – (q)
(b) A – (p), B – (r), C – (q)
(c) A – (r), B – (q), C – (p)
(d) A – (q), B – (p), C – (r)
Answer
A
Question. Match the columns
Column – I Column – II
(A) POCl3 (p) Contains four P – OH two P = O and one P – O – P
(B) H4P2O5 (q) Yellowish white chloride of phosphorus
reacts with moist air
(C) H4P2O6 (r) Contains four P – OH,two P = O and one P – P bond
(D) H4P2O7 (s) Colourless oily chloride of phosphorus reacts
with orthophosphoric acid
(a) A – (q), B – (s), C – (p), D – (r)
(b) A – (s), B – (q), C – (r), D – (p)
(c) A – (q), B – (s), C – (r), D – (p)
(d) A – (q), B – (r), C – (s), D – (p)
Answer
C
Question. Match the columns
Column – I Column – II
(A) XeF4 (p) Contains similar types of bonds
(B) XeOF4 (q) Contains maximum lone pair
(C) XeF2 (r) Square pyramidal geometry
(D) XeO3 (s) Contains one lone pair
(a) A – (p), B – (r, s), C – (p, q), D – (p, s)
(b) A – (r, s), B – (p), C – (r, s), D – (p, s)
(c) A – (p), B – (p, q), C – (r, s), D – (p, s)
(d) A – (p), B – (r, s), C – (p, s), D – (p, q)
Answer
A
Question.Match the columns
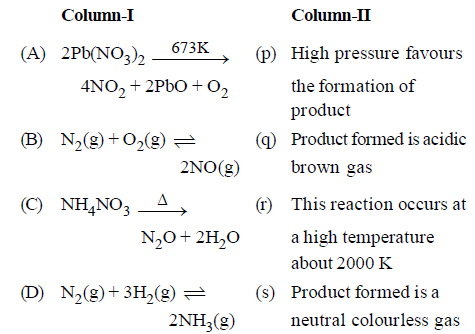
(a) A – (r, s), B – (q), C – (s), D – (p)
(b) A – (q), B – (r,s), C – (s), D – (p)
(c) A – (q), B – (s), C – (r, s), D – (p)
(d) A – (q), B – (r, s), C – (p), D – (s)
Answer
B


