Please refer to MCQ Questions Chapter 5 Periodic Classification of Elements Class 10 Science with answers provided below. These multiple-choice questions have been developed based on the latest NCERT book for class 10 Science issued for the current academic year. We have provided MCQ Questions for Class 10 Science for all chapters on our website. Students should learn the objective based questions for Chapter 5 Periodic Classification of Elements in Class 10 Science provided below to get more marks in exams.
Chapter 5 Periodic Classification of Elements MCQ Questions
Please refer to the following Chapter 5 Periodic Classification of Elements MCQ Questions Class 10 Science with solutions for all important topics in the chapter.
MCQ Questions Answers for Chapter 5 Periodic Classification of Elements Class 10 Science
Question: Which one of the following elements will form an acidic oxide?
a) An element with atomic number 7
b) An element with atomic number 3
c) An element with atomic number 12
d) None of these
Answer
A
Question: On the basis of following features identify correct option.
(i) These elements majorly forms acidic oxides.
(ii) These elements are majorly non-metals.
a) s-block elements b) p-block elements
c) d-block elements d) f-block elements
Answer
B
Question: The three elements calcium, strontium and barium form a triad.What is the basis of this grouping?
(i) Elements are in the increasing order of their atomic weights.
(ii) The atomic weight of the middle element is equal to the average of the atomic weight of extreme elements.
(iii) Elements in a triad have similar chemical properties.
a) Only (i) and (ii)
b) Only (ii) and (iii)
c) Only (i) and (iii)
d) (i), (ii) and (iii)
Answer
D
Question: Hydrogen has three isotopes 1H, 2H and 3H. On what basis these elements were placed in modern periodic table ?
a) Atomic mass
b) Atomic number
c) Both a) and (b
d) None of these
Answer
B
Question: If Cl, Br and I, are Dobereiner’s triad and the atomic masses of Cl and I are 35.5 and 127 respectively the atomic mass of Br is –
a) 162.5
b) 91.5
c) 81.25
d) 45.625
Answer
C
Question: An element ‘X’ is forming an acidic oxide. Its position in modern periodic table will be
a) Group 1 and Period 3
b) Group 2 and Period 3
c) Group 13 and Period 3
d) Group 16 and Period 3
Answer
D
Question: Newland could classify elements only upto –
a) copper
b) chlorine
c) calcium
d) chromium
Answer
C
Question: Noble gases were included in Mendeleev’s periodic table in the –
a) 1st group
b) 7th group
c) 8th group
d) none of these
Answer
D
Question: Mendeleev classified elements in –
a) increasing order of atomic groups
b) eight periods and eight groups
c) seven periods and nine groups
d) eight periods and seven groups
Answer
C
Question: In the modern periodic table which of the following does
not have appropriate position?
a) Transition elements
b) Inert gases
c) Inner transition elements
d) Halogens
Answer
C
Question: The correct order of first IE of C, N, O, F is –
a) F > O > N > C
b) C > N > O > F
c) O > N > F > C
d) F > N > O > C
Answer
D
Question: An element M has atomic number 9 and atomic mass 17. Its ion will be represented by –
a) M
b) M2+
c) M–
d) M2–
Answer
C
Question: The long form of periodic table consists of –
a) seven periods and eight groups
b) seven periods and eighteen groups
c) eight periods and eighteen groups
d) eighteen periods and eight groups
Answer
B
Question: The atoms of elements belonging to the same group of periodic table have the same –
a) number of protons
b) number of electrons
c) number of neutrons
d) number of electrons in the outermost shell
Answer
D
Question: Which of the following is the correct order of relative size?
a) I– > I+ > I
b) I– > I > I+
c) I > I+ > I–
d) I+ > I– > I
Answer
B
Question: The element with the smallest size in the group 13 is –
a) beryllium
b) carbon
c) aluminium
d) boron
Answer
D
Question: Elements belonging to the same group have similar properties because –
a) they have similar electronic configuration of the outermost shell.
b) their atomic numbers go on increasing as we move down the group.
c) all of them are metallic elements.
d) none of the above
Answer
A
Question: The most metallic element in the fifth period is –
a) silver
b) rubidium
c) gold
d) rhodium
Answer
B
Question: If the two members of a Dobereiner triad are chlorine and iodine, the third member of this triad is –
a) fluorine
b) bromine
c) sodium
d) calcium
Answer
B
Question: The element present in the 4th period is –
a) chlorine
b) iodine
c) fluorine
d) bromine
Answer
D
Question: According to Mendeleev periodic law, the properties of elements are periodic function of their –
a) atomic masses
b) atomic numbers
c) atomic volumes
d) densities
Answer
A
Question: The elements with atomic numbers 2, 10, 18, 36, 54 and 86 are all –
a) halogens
b) noble gases
c) noble metals
d) light metals
Answer
B
Question: The number of electrons in the valence shell is equal to its
a) atomic mass
b) group number
c) period number
d) atomic volume
Answer
B
Question: Which of the following set of elements is written in order of their increasing metallic character?
a) Be Mg Ca
b) Na Li K
c) Mg Al Si
d) C O N
Answer
A
Question: The non-metallic element present in the third period other than sulphur and chlorine is
a) oxygen
b) fluorine
c) nitrogen
d) phosphorus
Answer
D
Question: The property of an element in the periodic table depends on its, ________.
a) atomic size
b) atomic mass
c) electronic configuration
d) number of protons
Answer
C
Question: The family of elements having seven electrons in the outermost shell is
a) alkali metals
b) alkaline earth metals
c) halogens
d) noble gases
Answer
C
Question: Which of the following factors does not affect the metallic character of an element?
a) Atomic size
b) Ionisation potential
c) Electro negativity
d) Atomic radius
Answer
C
Question: Elements belonging to groups 1 to 17 are called
a) noble gases
b) normal elements
c) transition elements
d) inner transition elements
Answer
B
Question: The family of elements to which potassium belongs is
a) alkali metals
b) alkaline earth metals
c) halogens
d) noble gases
Answer
A
Question: At the end of each period the valence shell is
a) incomplete
b) half filled
c) singly occupied
d) completely filled
Answer
D
Question: The family of elements to which calcium belongs is
a) alkali metals
b) alkaline earth metals
c) halogens
d) noble gases
Answer
B
Question: A liquid non-metal is
a) phosphorous
b) mercury
c) bromine
d) nitrogen
Answer
C
Question: Four elements along a period have atomic number (11, 13, 16 and 17). The most metallic among these has an atomic number of
a) 11
b) 12
c) 16
d) 17
Answer
A
Question: The first alkali metal is
a) hydrogen
b) lithium
c) sodium
d) francium
Answer
B
Question: The modern periodic table is given by
a) Mendeleev
b) Einstein
c) Bohr
d) Mosley
Answer
D
Question: Lanthanides and actinides are also called
a) normal elements
b) transition elements
c) noble gases
d) inner transition elements
Answer
D
Question: The least reactive element in group 17 is
a) fluorine
b) chlorine
c) bromine
d) iodine
Answer
D
Question: A purple coloured solid halogen is
a) chlorine
b) bromine
c) iodine
d) astatine
Answer
C
Question: The number of shells in the elements of 3rd period is __________
a) 1
b) 2
c) 3
d) 0
Answer
C
Question: Six elements A, B, C, D, E and F have the following atomic numbers (A = 12, B = 17, C = 18, D =7, E = 9 and F = 11). Among these elements, the element, which belongs to the 3rd period and has the highest ionisation potential, is
a) A
b) B
c) C
d) F
Answer
C
Question: The valency of chlorine with respect to oxygen is
a) 1
b) 3
c) 5
d) 7
Answer
D
Question: A factor that affects the ionisation potential of an element is
a) atomic size
b) electron affinity
c) electro-negativity
d) neutron
Answer
A
Question: The statement that is not true about electron affinity is
a) It causes energy to be released
b) It causes energy to be absorbed
c) It is expressed in electron volts
d) It involves formation of an anion
Answer
A
Question: The element, which has the highest electron affinity in the 3rd period is
a) Na
b) Mg
c) Si
d) Cl
Answer
B
Question: Down a group, the electron affinity
a) increases
b) decreases
c) remains same
d) increases and then decreases
Answer
B
Question: Upto which element, the law of octaves was found to be applicable?
a) Oxygen
b) Calcium
c) Cobalt
d) Potassium
Answer
B
Question: The element, which has zero electron affinity in the 3rd period is
a) Al
b) P
c) Ar
d) S
Answer
C
Question: Which of the following statement(s) about the Modern Periodic Table are incorrect.
i) The elements in the Modern Periodic Table are arranged on the basis of their decreasing atomic number.
ii) The elements in the Modern Periodic Table are arranged on the basis of their increasing atomic masses.
iii) Isotopes are placed in adjoining group(s) in the Periodic Table.
iv) The elements in the Modern Periodic Table are arranged on the basis of their increasing atomic number
a) Only (i)
b) (i), (ii) and (iii)
c) (i), (ii) and (iv)
d) Only (iv) d
Answer
D
Question: Which one of the following depicts the correct representation of atomic radius(r)of an atom?
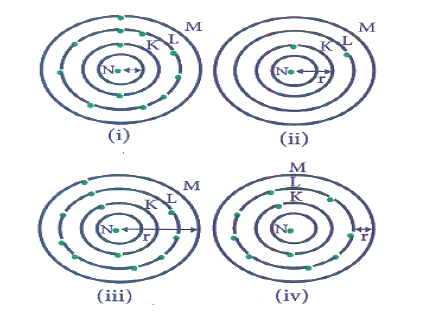
a) (i) and (ii)
b) (ii) and (iii)
c) (iii) and (iv)
d) (i) and (iv
Answer
B
Question: At present ___ elements are known to us.
a) 115
b) 116
c) 117
d) 118
Answer
A
Question: Periodic Law states that ‗the properties of elements are the periodic function of their
______________
a) atomic masses
b) structures
c) atomic weights
d) all of the above
Answer
A
Question: Number of elements those are naturally occurring.
a) 92
b) 93
c) 94
d) 95
Answer
A
Question: Dobereiner could identify only ______________triads from the elements known at that time
a) three
b) four
c) five
d) six
Answer
A
Question: Matter around us is present in the form of
a) elements
b) compounds
c) mixtures
d) all of the above
Answer
D
Question: It was assumed by Newlands that only ______________elements existed in nature and no more elements would be discovered in the future.
a) 36
b) 46
c) 56
d) 66
Answer
D
Question: Mendeleev could not assign a correct position to ______________. This was the first limitation
a) Oxygen
b) Carbon
c) Hydrogen
d) Calcium
Answer
C
Question: Mendeleev was a ______________chemist
a) American
b) English
c) French
d) Russian
Answer
A
Question: Mendeléev‘s Periodic Table contains vertical columns called
a) groups
b) periods
c) intervals
d) none of the above
Answer
A
Question: As per modern periodic law, ‗Properties of elements are a periodic function of their
a) atomic number
b) atomic mass
c) structure
d) none of the above
Answer
A
Question: Following is (are) the noble gas (es)
a) Helium
b) Neonc) Argon
d) All of the above
Answer
D
Question: Henry Moseley showed that the ______________of an element is a more fundamental property
a) atomic number
b) atomic mass
c) structure
d) none of the above
Answer
A
Question: The modern periodic table has ______________horizontal rows known as ‘periods‘
a) five
b) six
c) seven
d) eight
Answer
A
Question: In modern periodic table, the elements present in any one group have the same number of
a) valence electrons
b) atomic weight
c) atomic mass
d) all of the above
Answer
C
Question: The number of periods in the long form of the periodic table is
a) 6
b) 7
c) 10
d) 18
Answer
B
Question: Which of the following has maximum atomic size?
a) K
b) Ca
c) Al
d) P
Answer
A
Question:The law of octaves was proposed by
a) Newland
b) Doberiener
c) Lother Meyer
d) Mendleeve
Answer
A
Question: Which of the following is the most reactive halogen?
a) F
b) Cl
c) Br
d) I
Answer
A
Question: Which among the following elements has the largest atomic radii?
a) Na
b) Mg
c) K
d) Ca
Answer
C
Question: Modem Periodic Law was given by
a) Joule
b) Moseley
c) Klechkosky
d) Meyer
Answer
B
Question: The atomic number gives us the number of ______________in the nucleus of an atom.
a) electrons
b) neutrons
c) protons
d) any of the above
Answer
C
Question: In the Mendeleel’s periodic table properties of the elements are the periodic function of their
a) atomic weight
b) atomic number
c) atomic volume
d) none of these
Answer
A
Question: Law of octaves was given by
a) Meyer
b) Mendeleev
c) Moseley
d) Newland
Answer
D
Question: Which of the following did not find a place in Mendeleef’s periodic table?
a) Fr
b) Na
c) Ce
d) CI
Answer
A
Question: Which of the following elements has maximum metallic character?
a) Li
b) N
c) Na
d) P
Answer
C
Question:An element has configuration 2, 8, 1. It belongs to, ______________
a) 1 group and 3rd period
b) 3 group and 1st period
c) 1 group and 8th period
d) 17 group and 3rd period
Answer
A
Question: Which one of the following elements exhibit maximum number of valence electrons?
a) Na
b) Al
c) Si
d) P
Answer
D

We hope you liked the above provided MCQ Questions Chapter 5 Periodic Classification of Elements Class 10 Science with solutions. If you have any questions please ask us in the comments box below.


