Please refer to MCQ Questions Chapter 4 Carbon and Its Compound Class 10 Science with answers provided below. These multiple-choice questions have been developed based on the latest NCERT book for class 10 Science issued for the current academic year. We have provided MCQ Questions for Class 10 Science for all chapters on our website. Students should learn the objective based questions for Chapter 4 Carbon and Its Compound in Class 10 Science provided below to get more marks in exams.
Chapter 4 Carbon and Its Compound MCQ Questions
Please refer to the following Chapter 4 Carbon and Its Compound MCQ Questions Class 10 Science with solutions for all important topics in the chapter.
Question. Carbon exists in the atmosphere in the form of
(a) Only carbon monoxide
(b) Carbon monoxide in traces and carbon dioxide
(c) Only carbon dioxide
(d) Coal
Answer
C
Question. The heteroatoms present in CH3 ─ CH2 ─ O ─ CH2 ─ CH2Cl are:
(i) Oxygen
(ii) Carbon
(iii) Hydrogen
(iv) Chlorine
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iv)
Answer
D
Question . Which of the following represents saponification reaction?
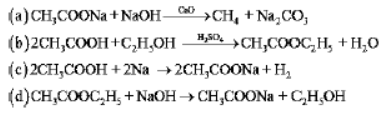
Answer
D
Question. The first member of alkyne homologous series is:
(a) Ethyne
(b) Ethene
(c) Propyne
(d) Methane
Answer
A
Question. Which of the following statements are usually correct for carbon compounds?
(i) They are good conductors of electricity
(ii) They are poor conductors of electricity
(iii) They have strong forces of attraction between their molecules
(iv) They do not have strong forces of attraction between their molecules
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer
D
Question. A molecule of ammonia (NH3) has
(a) Only single bonds
(b) Only double bonds
(c) Only triple bonds
(d) Two double bonds and one single bond
Answer
A
Question. Buckminsterfullerene is an allotropic form of
(a) Phosphorus
(b) Sulphur
(c) Carbon
(d) Tin
Answer
C
Question. Which of the following are correct structural isomers of C4H10?

(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i) and (ii)
(d) (iii) and (iv)
Answer
A
Question. In the following reaction, alkaline KMnO4 acts as:

(a) Reducing agent
(b) Oxidising agent
(c) Catalyst
(d) Dehydrating agent
Answer
B
Question. Oils on treating with hydrogen in the presence of palladium or nickel catalyst form fats. This is an example of
(a) Addition reaction
(b) Substitution reaction
(c) Displacement reaction
(d) Oxidation reaction
Answer
A
Question. In which of the following compounds -OH is the functional group?
(a) Butanone
(b) Butanol
(c) Butanoic
(d) Butanal
Answer
B
Question. The soap molecule has a
(a) Hydrophilic head and a hydrophobic tail
(b) Hydrophobic head and a hydrophilic tail
(c) Hydrophobic head and a hydrophobic tail
(d) Hydrophilic head and a hydrophilic tail
Answer
A
Question. Which of the following is the correct representation of electron dot structure of nitrogen?
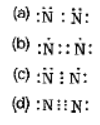
Answer
D
Question. Chlorine reacts with saturated hydrocarbons at room temperature in the
(a) absence of sunlight
(b) presence of sunlight
(c) presence of water
(d) presence of hydrochloric acid
Answer
B
Question. ‘Drinking alcohol’ is very harmful and it ruins the health. ‘Drinking alcohol’ stands for –
(a) drinking methyl alcohol
(b) drinking ethyl alcohol
(c) drinking propyl alcohol
(d) drinking isopropyl alcohol
Answer
B
Question. The treatment of acetic acid with lithium aluminium hydride produces –
(a) methanol
(b) ethanol
(c) ethanal
(d) methanal
Answer
B
Question. Fermentation of sugarcane juice produces
(a) Ethanol
(b) Ethanal
(c) Acetic acid
(d) Gluconic acid
Answer
A
Question. The OH group of an alcohol or the —COOH group of a carboxylic acid can be replaced by —Cl using :–
(a) phosphorus pentachloride
(b) hypochlorous acid
(c) chlorine
(d) hydrochloric acid
Answer
A
Question. Butanone is four-carbon compound with the functional group :
(a) carboxylic acid
(b) aldehyde
(c) ketone
(d) alcohol
Answer
C
Question. Percentage of nitrogen in urea (NH2CONH2) is
(a) 23.3%
(b) 46.7%
(c) 69.9%
(d) 11.66%
Answer
B
Question. Which compound represents the vinegar?
(a) HCOOH
(b) CH3CHO
(c) HCHO
(d) CH3COOH
Answer
D
Question. A & B both compounds give H2 gas with sodium. If A & B react in presence of acid catalyst then they form ethyl acetate. Thus, A & B would be –
(a) CH3COOH, CH3OH
(b) HCOOH, CH3COOH
(c) CH3COOH, C2H5OH
(d) C3H7COOH, C3H7OH
Answer
C
Question. Which one of the following statement is incorrect about graphite and diamond ?
(a) Graphite is smooth and slippery.
(b) Diamond is good conductor of heat.
(c) Graphite is a good conductor of electricity.
(d) Physical and chemical properties of graphite and diamond are same.
Answer
D
Question. During the cleansing action of soap dirt is surrounded by soap molecules. Soap molecule is like a tadpole which has a head and tail. These head and tail respectively are:
(a) hydrophobic and hydrophilic
(b) hydrophobic and hydrophobic
(c) hydrophilic and hydrophilic
(d) hydrophilic and hydrophobic
Answer
D
Question. In the soap micelles
(a) The ionic end of soap is on the surface of the cluster while the carbon chain is in the interior of the cluster
(b) Ionic end of soap is in the interior of the cluster and the carbon chain is out of the cluster
(c) Both ionic end and carbon chain are in the interior of the cluster
(d) Both ionic end and carbon chain are on the exterior of the cluster
Answer
A
Question. Pentane has the molecular formula C5H12. It has
(a) 5 covalent bonds
(b) 12 covalent bonds
(c) 16 covalent bonds
(d) 17 covalent bonds
Answer
C
Question. Structural formula of benzene is:
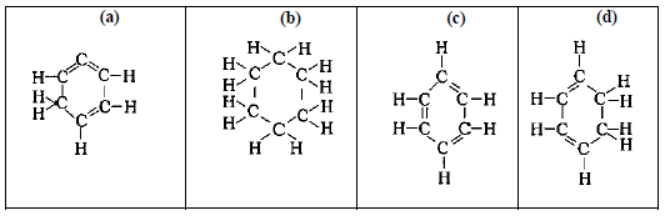
Answer
C
Question. Ethanol reacts with sodium and forms two products. These are
(a) Sodium ethanoate and hydrogen
(b) Sodium ethanoate and oxygen
(c) Sodium ethoxide and hydrogen
(d) Sodium ethoxide and oxygen
Answer
C
Question. The correct structural formula of butanoic acid is:
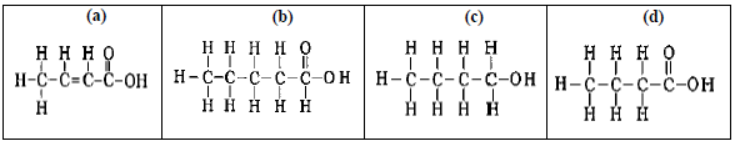
Answer
D
Question. Vinegar is a solution of:
(a) 50% – 60% acetic acid in alcohol
(b) 5% – 8% acetic acid in alcohol
(c) 5% – 8% acetic acid in water
(d) 50%- 60% acetic acid in water
Answer
D
Question. Mineral acids are stronger acids than carboxylic acids because
(i) Mineral acids are completely ionised.
(ii) Carboxylic acids are completely ionized
(iii) Mineral acids are partially ionisedCarboxylic acids are partially ionized
(iv) Carboxylic acids are partially ionized
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (ii)
(d) (iii) and (iv)
Answer
A
Question. Carbon forms four covalent bonds by sharing its four valence electrons with four univalent atoms, e.g. hydrogen. After the formation of four bonds, carbon attains the electronic configuration of
(a) Helium
(b) Neon
(c) Argon
(d) Krypton
Answer
B
Question. The correct electron dot structure of a water molecule is
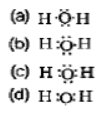
Answer
C
Question. Which of the following is not a straight chain hydrocarbon?
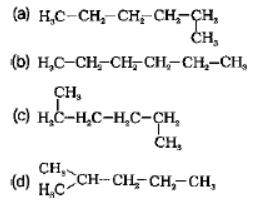
Answer
D
Question. Which among the following are unsaturated hydrocarbons?
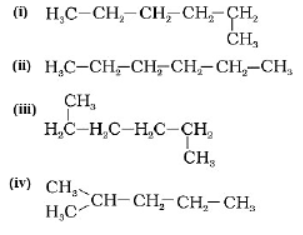
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (iii) and (iv)
Answer
D
Question. Structural formula of ethyne is
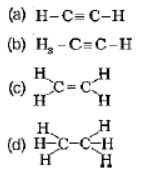
Answer
A
Question. Identify the unsaturated compounds from the following
(i) Propane
(ii) Propene
(iii) Propyne
(iv) Chloropropane
(a) (i) and (ii)
(b) (ii) and (iv)
(c) (iii) and (iv)
(d) (ii) and (iii)
Answer
D
Question. The name of the compound, CH3 ─ CH2 ─ CHO is:
(a) Propanal
(b) Propanone
(c) Ethanol
(d) Ethanal
Answer
A
(a) If both Assertion and Reason are correct and Reason is the correct explanation of Assertion.
(b) If both Assertion and Reason are correct, but Reason is not the correct explanation of Assertion.
(c) If Assertion is correct but Reason is incorrect.
(d) If Assertion is incorrect but Reason is correct.
Question. Assertion : Diamond and graphite are allotropes of carbon.
Reason : Some elements can have several different structural forms in the same physical state. These differing forms are called allotropes.
Answer
A
Question. Assertion : The correct IUPAC name for the compound is 2, 4 dimethyl hexane not 3, 5 dimethyl hexane
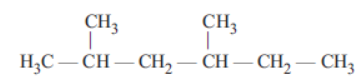
Reason: When the parent chain has two or more substitutents, numbering must be done in such a way that the sum of the locants on the parent chain is the lowest possible.
Answer
A
Question. Assertion : Carbon monoxide is extremely poisonous in nature.
Reason : Carbon monoxide is formed by complete combustion of carbon.
Answer
C
Question. Assertion : Carbon has ability to form long carbon chains.
Reason : Carbon has a unique property to form long straight and branched chains called catenation.
Answer
A
Question. Assertion: Ethanoic acid is called as glacial acetic acid.
Reason: On cooling it freezes to form ice-like flakes. They appear like a glaciers.
Answer
A
Fill in the Blanks :
Question. Valency of carbon in ethylene is …………… .
Answer
4
Question. The molecular mass of any two adjacent homologues differ by …………….. amu.
Answer
14
Question. The general formula of alcohols is ……………. .
Answer
CnH2n+1OH
Question. The functional group present in carboxylic acids is …………………
Answer
–COOH
Question. The purest form of carbon is ………………… .
Answer
diamond
True / False :
Question. Carbon is a versatile element.
Answer
True
Question. Ethanol is the first member of the alcohol homologous series.
Answer
False
Question. When hydrocarbons burn in air, carbon dioxide and hydrogen are produced with heat energy.
Answer
False
Question. If a hydrocarbon has double or triple covalent bond, it is saturated.
Answer
False
Question. Carbon forms covalent bonds with itself and other elements such as hydrogen, oxygen, sulphur, nitrogen and chlorine.
Answer
True
Question. The simplest saturated hydrocarbon is methane.
Answer
True
Question. Unsaturated hydrocarbons give addition reactions.
Answer
True

We hope you liked the above provided MCQ Questions Chapter 4 Carbon and Its Compound Class 10 Science with solutions. If you have any questions please ask us in the comments box below.


