Please refer to MCQ Questions Chapter 2 Acids Bases Salts Class 10 Science with answers provided below. These multiple-choice questions have been developed based on the latest NCERT book for class 10 Science issued for the current academic year. We have provided MCQ Questions for Class 10 Science for all chapters on our website. Students should learn the objective based questions for Chapter 2 Acids Bases Salts in Class 10 Science provided below to get more marks in exams.
Chapter 2 Acids Bases Salts MCQ Questions
Please refer to the following Chapter 2 Acids Bases Salts MCQ Questions Class 10 Science with solutions for all important topics in the chapter.
Question. Sodium hydroxide is a
(a) weak base
(b) weak acid
(c) strong base
(d) strong acid
Answer
C
Question. An aqueous solution turns red litmus solution blue. Excess addition of which of the following solution would reverse the change?
(a) Baking powder
(b) Lime
(c) Ammonium hydroxide solution
(d) Hydrochloric acid
Answer
D
Question. When copper oxide and dilute hydrochloric acid react, colour changes to
(a) white
(b) bluish-green
(c) blue-black
(d) black
Answer
B
Question. Na2CO3.10H2O is
(a) washing soda
(b) baking soda
(c) bleaching powder
(d) tartaric acid
Answer
A
Question. The equation shows the reaction of metal oxide with acid.
Metal oxide + Acid → X + Water
What is X?
(a) Salt
(b) Hydrogen
(c) Carbon dioxide
(d) Base
Answer
A
Question. An oxide of element P is added to an acid where it forms salt and water. The table shows the possible value of pH and the type of element before the reaction.
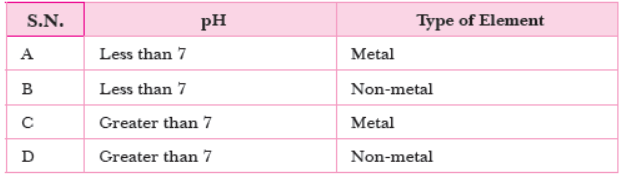
Which option is correct?
(a) A
(b) D
(c) C
(d) B
Answer
C
Question. At what temperature is gypsum heated to form Plaster of Paris?
(a) 90°C
(b) 100°C
(c) 110°C
(d) 120°C
Answer
B
Question. How many water molecules does hydrated calcium sulphate contain?
(a) 5
(b) 10
(c) 7
(d) 2
Answer
D
Question. Sodium hydroxide is used
(a) as an antacid
(b) in manufacture of soap
(c) as a cleansing agent
(d) in alkaline batteries
Answer
B
Question. Which of the following phenomena will occur when a small amount of acid is added to water?
(i) dilution (ii) neutralisation
(iii) salt formation (iv) ionization
(a) (i) and (iii)
(b) (i) and (iv)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer
B
Question. Brine is used for industrial production of
(a) NaOH
(b) KOH
(c) bleaching powder
(d) none of the above
Answer
A
Question. Sodium hydroxide turns phenolphthalein solution
(a) pink
(b) yellow
(c) colourless
(d) orange
Answer
A
Question. Which equation for the reaction between hydrochloric acid and sodium hydroxide is correct?
(a) HCl + NaOH → NaCl + H2O
(b) 2HCl + NaOH → 2NaCl + H2O
(c) 2HCl + NaOH → NaCl + 2H2O
(d) HCl + 2NaOH → Na2Cl + H2O
Answer
A
Question. A student placed 10 mL HCl and NaOH in two separate beakers as shown.
In beaker 1, 4 mL of NaOH is added whereas in beaker 2, 4 mL of HCl is added. The student notes the possible changes in pH in both solutions.
Which change in pH is correct?
(a) A
(b) B
(c) C
(d) D
Answer
C
Question. A solution of NaCl
(i) will turn red litmus blue (ii) will turn pH paper green
(iii) will turn blue litmus red (iv) will not affect litmus
(a) (i) and (ii)
(b) (i) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer
D
Question. Curd cannot be stored in
(i) Brass vessel (ii) Copper vessel
(iii) Steel (iv) Bronze
(a) (i), (ii), (iii)
(b) (ii), (iii), (iv)
(c) (i), (ii), (iv)
(d) (i), (iii), (iv)
Answer
C
Question. Sodium carbonate is a basic salt because it is a salt of
(a) strong acid and strong base.
(b) weak acid and weak base.
(c) strong acid and weak base.”
(d) weak acid and strong base.
Answer
D
Question. Which of the following is used for dissolution of gold ?
(a) Hydrochloric acid
(b) Sulphuric acid
(c) Nitric acid
(d) Aqua regia
Answer
D
Question. Which of the following are present in a dilute aqueous solution of hydrochloric acid?
(a) H3O+ + Cl–
(b) H3O+ + OH–
(c) Cl– + OH–
(d) unionised HCl
Answer
A
Question. NaHCO3, formed by reaction of
(a) NaOH + H2CO3
(b) NaCl + H2CO3
(c) Na2CO3 + HCl
(d) NaOH + Na2CO3
Answer
A
Question. pH of H2O is
(a) 7
(b) 8
(c) 9
(d) 10
Answer
Question. The acid used for the manufacture of fertilizers and explosives is
(a) nitric acid
(b) sulphuric acid
(c) phosphoric acid
(d) hydrochloric acid
Answer
A
Question. Which acid is found in bee sting?
(a) Citric acid
(b) Formic acid
(c) Tartaric acid
(d) Nitric acid
Answer
B
Question. A student did an activity in which he added sodium bicarbonate to hydrochloric acid. It forms the carbon dioxide gas. The gas released is passed through lime water. What change will be observed in lime water?
(a) The colour of solution becomes red.
(b) White precipitate is formed.
(c) The solution becomes colourless.
(d) Bubbles are formed.
Answer
B
Question. A metal carbonate reacts with a solution X which forms a salt, water, and a gas Y. What are X and Y?
(a) X: sodium hydroxide; Y: carbon dioxide
(b) X: sodium hydroxide; Y: hydrogen
(c) X: hydrochloric acid; Y: carbon dioxide
(d) X: hydrochloric acid; Y: hydrogen
Answer
C
Question. Ag2S reacts with H2SO4 to form
(a) AgSO4
(b) Ag2SO4 + H2S
(c) Ag2O + H2S
(d) AgOH + H2S
Answer
B
Question. Lime water reacts with chlorine to form
(a) CaCl2
(b) CaOCl2
(c) Ca(ClO3)2
(d) CaO2Cl2
Answer
B
Question. The ratio of the water molecule in Plaster of Paris and Gypsum is
(a) 3:1
(b) 1:3
(c) 1:4
(d) 4:3
Answer
C
Question. CaOCl2 will liberate Cl2 gas in presence of
(i) CO2 (ii) HCl
(iii) CO (iv) NO
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (ii) and (iv)
Answer
A
Question. Egg shell is made up of
(a) CaCO3
(b) CaO
(c) Ca(OH)2
(d) CaCl2
Answer
D
Question. What happens when an alkali is mixed with water?
(a) Heat is evolved
(b) Heat is absorbed
(c) Concentration of acid increases
(d) All of the above
Answer
A
Question. Which of the following is alkali?
(a) Sodium hydroxide
(b) Calcium carbonate
(c) Copper carbonate
(d) Carbonic acid
Answer
A
Question. Baking powder is
(a) sodium carbonate + sodium tartarate
(b) sodium bicarbonate + sodium tartarate
(c) sodium bicarbonate + tartaric acid
(d) sodium carbonate + sodium benzoate
Answer
C
Question. Chemical formula of washing soda is
(a) Na2CO3.7H2O
(b) Na2CO3.5H2O
(c) Na2CO3.2H2O
(d) Na2CO3.10H2O
Answer
D
Question. Which of the following is not a acidic salt?
(a) CuSO4
(b) NH4Cl
(c) FeCl3
(d) CH3COONa
Answer
D
Question. What happens when an acid is added to vanilla?
(a) Colour of vanilla changes into red
(b) Vanilla becomes colourless
(c) Vanilla loses its smell
(d) Nothing happens
Answer
Question. What is the original colour of phenolphthalein solution which is an indicator?
(a) Colourless
(b) Red
(c) Pink
(d) Violet
Answer
A
Question. Gastric juice contains HCl which is one example of
(a) inorganic acid
(b) organic acid
(c) soft organic acid
(d) strong inorganic acid
Answer
D
Question. When milk of magnesia reacts with acetic acid it produces
(a) basic salt
(b) acidic salt
(c) neutral salt
(d) complex salt
Answer
C
Question. When base reacts with the non-metal oxide
(a) it neutralizes each other
(b) it creates fire
(c) it produces acidic salts
(d) it produces basic salts
Answer
A
Question. Many salts absorbs water from atmosphere. This property is called
(a) deliquescence
(b) efflorescence
(c) hydration
(d) addition
Answer
A
Question. An aqueous solution with pH = 1 is
(a) strongly acidic
(b) strongly basic
(c) neutral
(d) weakly acidic
Answer
A
Question. Corrosive effect on the skin is caused by
(a) acids and bases
(b) bases and salts
(c) water
(d) mercury
Answer
A
Question. When dilute sulphuric acid is added to a solid X, a gas Y is formed along with the formation of the salt of the solid. What could be X and Y?
(a) X: carbon; Y: hydrogen
(b) X: zinc; Y: hydrogen
(c) X: zinc; Y: oxygen
(d) X: copper; Y: oxygen
Answer
C
Question. When a base reacts with a metal, it forms a salt and hydrogen gas is released. By what method the presence of hydrogen can be detected?
(a) By methyl orange
(b) By water
(c) By litmus paper
(d) By a burning candle
Answer
D
Question. Rubbing of which does give relief from pain in the case of bee sting?
(a) Dilute hydrochloric acid
(b) Dilute nitric acid
(c) Tooth paste
(d) Alkali
Answer
C
Question. Which of the following is called alkali?
(a) Water soluble base
(b) Water insoluble base
(c) Carbonate of metals
(d) Oxides of metals
Answer
A
Question. What happens when an acid react with base?
(a) Acid neutralizes base
(b) Water is formed
(c) A salt is formed
(d) All of the above
Answer
D
Question. Which of the following is an olfactory indicator?
(a) Turmeric
(b) Onion
(c) Litmus
(d) All of the above
Answer
B
Question. What happens when a base is added to vanilla?
(a) Colour of vanilla changes into red
(b) Vanilla becomes colourless
(c) Vanilla loses its smell
(d) Nothing happens
Answer
C
Question. A visually challenged student, has to perform a lab test to detect the presence of acid in a given solution. The acid-base indicator preferred by him will be:
(a) Blue litmus
(b) Clove oil
(c) Red cabbage extract
(d) Hibiscus extract
Answer
B
Question. Which of the following substances will not give carbon dioxide on treatment with dilute acid?
(a) Marble
(b) Limestone
(c) Baking soda
(d) Lime
Answer
D
Question. Equal volumes of hydrochloric acid and sodium hydroxide solutions of same concentration are mixed and the pH of the resulting solution is checked with a pH paper. What would be the colour obtained? (You may use colour guide in the figure given below).
(a) Red
(b) Yellow
(c) Yellowish green
(d) Blue
OR
Which of the following is/are true when HCl(g) is passed through water:
(i) It does not ionise in the solution as it is a covalent compound
(ii) It ionises in the solution
(iii) It gives both hydrogen and hydroxyl ion in the solution
(iv) It forms hydronium ion in the solution due to the combination of hydrogen ion with water molecule
(a) Only (i)
(b) Only (iii)
(c) (ii) and (iv)
(d) (iii) and (iv)
Answer
C
Question. Which of the following phenomena occur, when a small amount of acid is added to water?
(i) Ionisation (ii) Neutralisation
(iii) Dilution (iv) Salt formation
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (ii) and (iv)
Answer
B
Question. A sample of soil is mixed with water and allowed to settle. The clear supernatant solution turns the pH paper yellowish-orange.
Which of the following would change the colour of this pH paper to greenish-blue:
(a) Lemon juice
(b) Vinegar
(c) Common salt
(d) An antacid
Answer
D
Question. If a few drops of a concentrated acid accidentally spills over the hand of a student, what should be done?
(a) Wash the hand with a saline solution
(b) Wash the hand immediately with plenty of water and apply a paste of sodium hydrogen carbonate
(c) After washing with plenty of water, apply a solution of sodium hydroxide on the hand
(d) Neutralise the acid with a strong alkali
Answer
B
Question. What is observed when we pour a drop of acetic acid first on red and then on blue litmus papers?
(a) Red litmus paper becomes colourless and blue litmus paper remains blue.
(b) Red litmus paper turns blue and blue litmus paper remains blue.
(c) Red litmus paper remains red and blue litmus paper turns red.
(d) Red litmus paper turns blue and blue litmus paper turns red.
Answer
C
Question. Which one of the following can be used as an acid-base indicator by a visually impaired student?
(a) Litmus
(b) Turmeric
(c) Vanilla essence
(d) Petunia leaves
Answer
C
Question. Which of the following substances will not give carbon dioxide on treatment with dilute acid?
(a) Marble
(b) Limestone
(c) Baking soda
(d) Lime
Answer
D
Question. Which of the following statements is correct about an aqueous solution of an acid and a base?
(i) Higher the pH, stronger the acid
(ii) Higher the pH, weaker the acid
(iii) Higher the pH, stronger the base
(iv) Lower the pH, weaker the base
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (ii) and (iv)
Answer
A
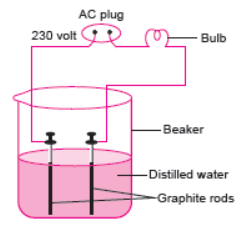
Question. In an attempt to demonstrate electrical conductivity through an electrolyte, the following apparatus (figure) was set up.
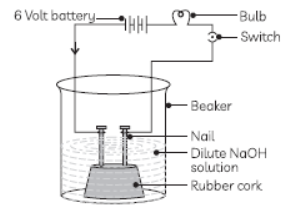
Which among the following statement(s) is/are correct?
(i) Bulb will not glow because electrolyte is not acidic.
(ii) Bulb will glow because NaOH is a strong base and furnishes ions for conduction.
(iii) Bulb will not glow because circuit is incomplete.
(iv) Bulb will not glow because it depends upon the type of electrolytic solution.
(a) (i) and (iii)
(b) (ii) and (iv)
(c) Only (ii)
(d) Only (iv)
Answer
C
Question. To protect tooth decay we are advised to brush our teeth regularly. The nature of the toothpaste commonly used is:
(a) Acidic
(b) Neutral
(c) Basic
(d) Corrosive
Answer
C
Question. Which of the following is not a mineral acid?
(a) Hydrochloric acid
(b) Citric acid
(c) Sulphuric acid
(d) Nitric acid
Answer
B
Question. Identify the correct representation of reaction occurring during chloralkali process.
(a) 2NaCl(l) + 2H2O(l) → 2NaOH(l) + Cl2(g) + H2(g)
(b) 2NaCl(l) + 2H2O(aq) → 2NaOH(aq) + Cl2(g) + H2(aq)
(c) 2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(aq) + H2(aq)
(d) 2NaCl(aq) + 2H2O(l) → 2NaOH(aq) + Cl2(g) + H2(g)
Answer
D
Question. If 10 mL of H2SO4 is mixed with 10 mL of Mg(OH)2 of the same concentration, the resultant solution will give the following colour with universal indicator:
(a) Red
(b) Yellow
(c) Green
(d) Blue
Answer
C
Question. Which of the following salts does not contain water of crystallisation?
(a) Blue vitriol
(b) Baking soda
(c) Washing soda
(d) Gypsum
Answer
B
Question. Equal volumes of hydrochloric acid and sodium hydroxide solutions of the same concentration are mixed and the pH of the resulting solution is checked with a pH paper.
What would be the color obtained? (You may use color guide in the figure given below).
(a) Red
(b) Yellow
(c) Yellowish green
(d) Blue (Image 50)
Answer
C
Question. Which of the following statements is true for acids?
(a) Bitter and change red litmus to blue.
(b) Sour and change red litmus to blue.
(c) Sour and change blue litmus to red.
(d) Bitter and change blue litmus to red.
Answer
C
Question. Zinc granules on treating with an acid X, form zinc sulphate (ZnSO4) salt along with the evolution of a gas Y, which burns with a pop sound when brought near to a burning candle. Identify acid X and gas evolved Y.
(a) X-sulphuric acid and Y-oxygen gas
(b) X-hydrochloric acid and Y-oxygen gas
(c) X-sulphuric acid and Y-hydrogen gas
(d) X-hydrochloric acid and Y-hydrogen gas
Answer
C
Assertion-Reason Questions
In each of the following questions, a statement of Assertion (A) is given followed by a corresponding statement of Reason (R). Select the correct answer to these questions from the codes (a), (b), (c) and (d) as given below:
(a) Both (A) and (R) are true and (R) is the correct explanation of the (A).
(b) Both (A) and (R) are true, but (R) is not the correct explanation of the (A).
(c) (A) is true, but (R) is false.
(d) (A) is false, but (R) is true.
Question. Assertion (A) : HCl gas does not change the colour of dry blue litmus paper.
Reason (R) : Acids always produce hydrogen ions.
Answer
C
Question. Assertion (A) : Generally, the colour of indicators changes in particular pH range.
Reason (R) : Indicators are weak acids or weak base and exhibit different colours in molecular form and ionic form.
Answer
A
Question. Assertion (A) : Acetic acid is monobasic acid.
Reason (R) : Acetic acid is a weak acid and produces less concentration of H+ ions.
Answer
B
Question. Assertion (A) : A stain of curry on a white cloth becomes reddish brown when scrubbed with soap.
Reason (R) : Litmus is a natural indicator.
Answer
B
Question. Assertion (A) : On passing carbon dioxide gas through lime water, following reaction takes place :
CaCO3(s) + CO2(g) + H2O(l) → Ca(HCO3)2(aq).
Reason (R) : Metal carbonates react with acids to give a corresponding salt.
Answer
D
Question. Assertion (A) : Zinc reacts with sodium hydroxide solution and
hydrogen gas is evolved.
Reason (R) : All metals react with bases toevolve hydrogen gas.
Answer
C
Question. Assertion (A) : While diluting an acid, water is slowly added to acid with constant stirring.
Reason (R) : The process of dissolving an acid in water is a highly exothermic reaction.
Answer
D
Question. Assertion (A) : When copper oxide is added to dilute hydrochloric acid,the colour of the solution becomes blue-green.
Reason (R) : Copper (II) chloride is formed.
Answer
A
Question. Assertion (A) : Metal oxides are acidic in nature.
Reason (R) : Calcium hydroxide reacts with carbon dioxide to form a salt and water.
Answer
D
Question. Assertion (A) : When copper sulphate crystals are heated in a dry boiling tube, they turn white.
Reason (R) : Water of crystallization is the number of water molecules present in one formula unit of a salt.
Answer
B
(a) If both Assertion and Reason are correct and Reason is the correct explanation of Assertion.
(b) If both Assertion and Reason are correct, but Reason is not the correct explanation of Assertion.
(c) If Assertion is correct but Reason is incorrect.
(d) If Assertion is incorrect but Reason is correct.
Question. Assertion : Dry HCl gas does not change the colour of blue litmus paper to red.
Reason : Dry HCl gas is strongly basic.
Answer
C
Question. Assertion : All alkalis are bases but all bases are not alkali.
Reason : Water soluble bases are alkali.
Answer
A
Question. Assertion : Sodium hydrogen carbonate is used in fire extinguisher.
Reason : Sodium hydrogen carbonate is a mild base.
Answer
B
Question. Assertion : Salts are the products of an acid-base reaction.
Reason : Salt may be acidic or basic.
Answer
B
Question. Assertion : Aqueous solution of ammonium nitrate turns blue litmus red.
Reason : Ammonium nitrate is salt of strong acid and strong base.
Answer
C
Question. Assertion : Magnesium hydroxide is used as antacid.
Reason : Magnesium hydroxide is a strong base.
Answer
C
Fill in the Blanks :
Question. Oxy acids contains …….. atoms in addition to hydrogen atom.
Answer
Oxygen
Question. An alkali reacts with ammonium salts to produce corresponding salt, water and evolve ……………… .
Answer
Ammonia
Question. When an acid reacts with a metal, …………. gas is evolved and a corresponding …………. is formed.
Answer
Hydrogen, salt
Question. Zn(OH)2 is a …………….. base.
Answer
Diacidic
Question. An acid that contains more than one acidic hydrogen atom is called a ………….. .
Answer
Polyprotic acid
True / False :
Question. Acidic nature of a substance is due to the formation of H+(aq) ions in solution.
Answer
True
Question. The colour of caustic soda turns pink when phenolphthalein is added.
Answer
True
Question. Solution of sodium hydrogen carbonate is alkaline in nature.
Answer
True
Question. Hydrogen chloride gas turns the blue litmus red.
Answer
False
Question. Sodium hydrogen carbonate is used in fire extinguisher.
Answer
True

We hope you liked the above provided MCQ Questions Chapter 2 Acids Bases Salts Class 10 Science with solutions. If you have any questions please ask us in the comments box below.


