Please refer to MCQ Questions Chapter 7 The p – Block Elements Class 12 Chemistry with answers provided below. These multiple-choice questions have been developed based on the latest NCERT book for class 12 Chemistry issued for the current academic year. We have provided MCQ Questions for Class 12 Chemistry for all chapters on our website. Students should learn the objective based questions for Chapter 7 The p – Block Elements in Class 12 Chemistry provided below to get more marks in exams.
Chapter 7 The p – Block Elements MCQ Questions
Please refer to the following Chapter 7 The p – Block Elements MCQ Questions Class 12 Chemistry with solutions for all important topics in the chapter.
Question. Which gas is evolved by the treatment of magnesium with very dilute solution of HNO3?
(a) N2
(b) NO2
(c) H2
(d) H2O
Answer
C
Question. Which of the following is not correct?
(a) Ammonia is used as refrigerant
(b) A mixture of Ca(CN)2 and C is known as nitrolirn
(c) A mixture of Ca(H2PO4 )2 and CaSO4 . 2H2O is known as superphosphate of lime
(d) Hydrolysis of NCl3 gives NH3 and HOCl
Answer
B
Question. Dinitrogen pentoxide (N2O5 ), a colourless solid, is prepared by
(a) heating NH4NO2 with an excess of oxygen
(b) dehydrating HNO3 with Cao
(c) dehydrating HNO3 with P4O10
(d) heating a mixture of HNO2 and Ca(NO3)2
Answer
C
Question. Among the following observations, the correct one that differentiates between SO32- and SO42- is
(a) both form precipitate with BaCl2 , SO32- dissolves in HCl but SO42- does not
(b) SO32- forms precipitate with BaCl2 , SO42- does not
(c) SO42- forms precipitate with BaCl2, SO32- does not
(d) both form precipitate with BaCl2, SO42- dissolves in HCl but SO32- does not
Answer
A
Question. PCl3 and cold water reacts to produce which of the following ?
(a) H3PO3
(b) H3PO2
(c) H4P2O7
(d) H3PO4
Answer
A
Question. Which of the following is kept in water ?
(a) White phosphorus
(b) Sodium
(c) Potassium
(d) Calcium
Answer
A
Question. White phosphorus is
(a) a monoatomic gas
(b) P4, a tetrahedral solid
(c) P8, a crown
(d) a linear diatomic molecule
Answer
B
Question. Consider the following statements.
I. NCl5 does not exist while PCl5 does.
II. Both O2+ and NO are paramagnetic.
III. The three C—O bonds are not equal in carbonate ion.
IV. Lead prefers to form tetravalent compound.
Which of the above statements are incorrect ?
(a) I and m
(b) I, III and IV
(c) II an III
(d) III and IV
Answer
C
Question. Which phosphorus reacts with KOH solution to produce phosphine gas ?
(a) White phosphorus
(b) Red phosphorus
(c) Both (a) and (b)
(d) None of the above
Answer
A
Question. Incorrect statement for pyrophosphoric acid H4P2O5 is
(a) it contains P in + 5 oxidation state
(b) it is dibasic acid
(c) it is strongly reducing in nature
(d) it contains one P—O—P bond
Answer
A
Question. A crystalline solid X reacts with dil. HCl to liberate a gas Y. Y decolourises acidified KMnO4 . When a gas Z is slowly passed into an aqueous solution of Y, colloidal sulphur is obtained X and Z could be respectively
(a) Na2S ,SO3
(b) Na2SO4 ,H2S
(c) Na2SO3 ,H2S
(d) Na2SO4 ,SO2
Answer
C
Question. Conversion of oxygen into ozone is non-spontaneous at
(a) all temperature
(b) high temperature
(c) room temperature
(d) low temperature
Answer
B
Question. Which of the following oxide does not form acidic aqueous solution ?
(a) N2O3
(b) NO2
(c) N2O5
(d) NO
Answer
D
Question. Which blue-liquid is obtained on reacting amounts of two gases at – 30° C?
(a) N2O
(b) N2O3
(c) N2O4
(d) N2O5
Answer
B
Question. When heated NH3 is passed over CuO, gas evolved is
(a) N2
(b) N2O
(c) HNO3
(d) NO2
Answer
A
Question. When the substances CCI4, CH4 and CF4 are arranged in order of increasing boiling point, what is the correct order ?
(a) CCI4, CF4, CH4
(b) CH4, CF4, CCI4
(c) CF4, CCI4, CH4
(d) CF4, CH4, CCI4
Answer
B
Question. (SiH3 )3 N is
(a) planar
(c) octahedral
(b) pyramidal
(d) tetrahedral
Answer
A
Question. The metal which does not form ammonium nitrate by reaction with dilute nitric acid is
(a) Al
(b) Fe
(c) Pb
(d) Mg
Answer
C
Question. The number of hydrogen atom(s) attached to phosphorus atom in hypophosphorus acid is
(a) three
(b) one
(c) two
(d) zero
Answer
C
Question. Which requires catalyst ?
(a) S + O2 → SO2
(b) 2SO2 + O2 → 2SO3
(c) C + O2 → CO2
(d) All of the above
Answer
B
Question. Which of the following is tetrabasic acid?
(a) Orthophosphorus acid
(b) Orthophosphric acid
(c) Meta phosphoric acid
(d) Pyrophosphoric acid
Answer
D
Question. When plants and animals decay, the organic nitrogen is converted into inorganic nitrogen. The inorganic nitrogen is in the form of
(a) ammonia
(b) elements ofnitrogen
(c) nitrates
(d) nitrides
Answer
A
Question. The correct statements (s) about O3 is (are)
(a) O—Obond lengths are equal
(b) thermal decomposition of O3 is endothermic
(c) O3 is diamagnetic in nature
(d) O3 has a bent structure
Answer
A,C,D
Question. In which of these processes platinum is used as a catalyst?
(a) Oxidation of ammonia to form HNO3
(b) Hardening of oils
(c) Production of synthetic rubber
(d) Synthesis of methanol
Answer
A
Question. In the manufacture of s ulphuric acid by contact process, Tyndall box is used to
(a) filter dust particles
(b) remove impurities
(c) convert SO2 to SO3
(d) test the presence of dust particles
Answer
D
Question. The structure of 01thophosphoric acid is

Answer
A
Question. Which of the following is a tribasic acid ?
(a) H3PO4
(b) HPO3
(c) H4P2O7
(d) H4P2O6
Answer
A
Question. Iron is dropped in dil. HNO3, it gives
(a) ferric nitrate
(b) ferric nitrate and NO2
(c) ferrous nitrate and ammonium nitrate
(d) ferrous nitrate and nitric oxide
Answer
C
Question. Which one of the following pairs of reactants does not form oxygen when they react with each other ?
(a) F2, NaOH solution (hot, cone.)
(b) F2, H2O
(c) Cl2 , NaOH solution (cold, clilute)
(d) CaOCl2 , H2SO4 (dilute, small amount)
Answer
C
Question. In Ostwald process of manufacturing of HNO3 , catalyst used is
(a) Mo
(b) Fe
(c) Mn
(d) Pt
Answer
D
Question. Which is most thermodynamically stable allotropic form of phosphorus ?
(a) Red
(b) White
(c) Black
(d) Yell ow
Answer
C
Question. The atomicity of sulphur in rhombic sulphur is
(a) 1
(b) 2
(c) 4
(d) 6
(e) 8
Answer
E
Question. When H2S gas is passed through nitric acid, the product is
(a) rhombic S
(b) prismatic S ( colloidal)
(c) amorphous S
(d) monoclinic S
(e) plastic S
Answer
B
Question. The substance which does not liberate oxygen on treatment with ozone is
(a) PbS
(b) HCl
(c) SO2
(d) Hg
Answer
C
Question. Which of the following statements regarding sulphur is incorrect ?
(a) SO2 molecule is paramagnetic
(b) The vapour at 200°C consists mostly of S8 rings
(c) At 600°C the gas main] y consists of S2 molecules
(d) The oxidation state of sulphur is never less than+ 4 in its compounds
Answer
D
Question. Which one of the following is not true at room temperature and pressure?
(a) P4O10 is a white solid
(b) SO2 is a colourless gas
(c) SO3is a colourless gas
(d) NO2 is brown gas
Answer
C
Question. The correct order ofreducing abilities of hydrides of V group elements is
(a) NH3 < PH3 < AsH3 < SbH3 < BiH3
(b) NH3 > PH3 > AsH3 > SbH3 > BiH3
(c) NH3 < PH3 > AsH3 > SbH3 > BiH3
(d) SbH3 > BiH3 > AsH3 > NH3 > PH3
Answer
A
Question. Oleum is chemically
(a) H2SO3
(b) H2SO5
(c) H2S2O7
(d) H2S2O8
Answer
C
Question. Concentrated sulphuric acid can be reduced by
(a) NaCl
(b) NaF
(c) NaOH
(d) NaNO3
(e) NaBr
Answer
E
Question. Reaction of PCl3 and PhMgBr would give
(a) bromobenzene
(b) chlorobenzene
(c) triphenylphosphine
(d) dichlorobenzene
Answer
C
Question. Which compound does not has S—S bond ?
(a) Na2S2O4
(b) Na2S4O6
(c) Na2S2O3
(d) Na2S2O7
Answer
D
Question. The function of Fe(OH)3 in the contact process is
(a) to remove arsenic impurity
(b) to detect colloidal impurity
(c) to remove moisture
(d) to remove dust particles
Answer
A
Question. The ore that is concentrated by the froth floatation process is
(a) cryolite
(b) cuprite
(c) calamine
(d) chalcopyrites
Answer
D
Question. A gas, that relights glowing splinter, is
(a) H2
(b) O2
(c) N2
(d) NO2
Answer
B
Question. Which element is not considered as ‘chalcogens’?
(a) Selenium
(b) Oxygen
(c) Sulphur
(d) Polonium
Answer
D
Question. Which of the following salt would give SO2 with hot and dil. H2SO4 and also decolourises Br2 water?
(a) Na2SO3
(b) NaHSO4
(c) Na2SO4
(d) Na2S
Answer
A
Question. Excess of PCl5 reacts with cone. H2SO4 giving
(a) chlorosulphonic acid
(b) thionyl chloride
(c) sulphuryl chloride
(d) sulphurous acid
Answer
C
Question. In presence of moisture, SO2 can
(a) act as oxidant
(b) act as reductant
(c) gain electron
(d) not act as reductant
Answer
B
Question. Ozone is used for purifying water because
(a) it dissociates and release oxygen
(b) do not leave any foul smell like chlorine
(c) kills bacteria, cyst, fungi and acts as a biocide
(d) All of the above
Answer
C
Question. Oxalic acid when heated with cone. H2SO4 , gives
(a) H2O2 and CO2
(b) CO and CO2
(c) H2O2 and CO
(d) CO2 and H2S
Answer
B
Question. Copper turnings on heating with cone. H2SO4 produce
(a) H2S
(b) O2
(c) SO3
(d) SO2
Answer
D
Question. Sulphur does not exist as S2 molecule because
(a) it is less electronegative
(b) it is not able to constitute pπ-pπ bonds
(c) it has ability to exhibit catenation
(d) of tendency to show variable oxidation states
Answer
B
Question. H2 Sis not a/an
(a) reducing agent
(b) acidic
(c) oxidising agent
(d) None of these
Answer
C
Question. In the laboratory H2S gas is prepared by using black lumps and dil. H2SO4 . The black lumps are
(a) FeSO4
(b) MnO2
(c) FeS
(d) FeSO3
Answer
C
Question. Polyanion formation is maximum in
(a) nitrogen
(b) sulphur
(c) oxygen
(d) boron
Answer
B
Question. When cone. H2SO4 is heated with P2O5 , the acid is converted into
(a) sulphur trioxide
(b) sulphur dioxide
(c) sulphur
(d) a mixture of sulphur dioxide and sulphur trioxide
Answer
A
Question. Which among the following statements are correct ?
I. Carbon monoxide is neutral whereas SO3 is acidic.
II. Potassium oxide is basic whereas nitrous oxide is acidic.
III. Aluminium and zinc oxides are amphoteric.
IV. Sulphur trioxide is aciclic whereas phosphorus pentaoxide is basic.
V. Carbon dioxide is neutral whereas sulphur d ioxide is amphoteric.
(a) II and III
(b) I and IV
(c) I and III
(d) II and IV
(e) III and V
Answer
C
Question. Select the incon-ect statement among the following.
(a) O3 is used as germicide for purification of air
(b) In O3 , O—O bond length is identical with that of molecular oxygen.
(c) O3 molecule is angular in shape.
(d) O3 is an oxidising agent.
Answer
B
Question. Olewn is
(a) castor oil
(b) oil of vitriol
(c) fuming H2SO4
(d) None of these
Answer
C
Question. Which of the following is not correct?
Silent electric
(a) 302 ⇔ 2O3 ; ΔH = – 284.5 kJ
discharge
(b) Ozone undergoes addition reaction with unsaturated carbon compounds
(c) Sodium thiosulphate reacts with I2 to form sodium tetratbionate and sodium iodide
(d) Ozone oxidises lead sulphide to lead sulphate
Answer
A
Question. At T (K.), 100 L of dry oxygen is present in a sealed container. It is subjected to silent electric discharge, till the volumes of oxygen and ozone become equal. What is the volume (in litres) of ozone formed at T (K)?
(a) 50
(b) 60
(c) 30
(d) 40
Answer
D
Question. Which of the following is most acidic hydride ofnitrogen?
(a) N3H
(b) NH3
(c) N2H4
(d) Both N3H and NH3
Answer
A
Question. Which is tribasic acid ?
(a) H3PO2
(b) H3PO4
(c) H4P2O7
(d) H3PO3
Answer
B
Question. Which of the following statement is true?
(a) H3PO3 is a stronger acid than H2SO3
(b) In aqueous medium HF is a stronger acid than HCl
(c) HCIO4 is a weaker acid than HCIO3
(d) HNO3 is a stronger acid than HNO2
Answer
D
Question. Which of the following is the correct order of increasing enthalpy of vaporisation ?
(a) NH3 < PH3 < AsH3
(b) AsH3 < PH3 < NH3
(c) PH3 < AsH3 < NH3
(d) NH3 < AsH3 < PH3
Answer
C
Question. Which of the following is not oxiclised by O3?
(a) KI
(b) FeSO4
(c) KMnO4
(d) K2MnO4
Answer
C
Question. Which one is con-ect statement ?
(a) Basicity of H3PO4 and H3PO3 is 3 and 3 respectively
(b) Acidity of H3PO4 and H3PO3 is 3 and3 respectively
(c) Acidity of H3PO4 and H3PO3 is 3 and2 respectively
(d) Basicity of H3PO4 and H3PO3 is 3 and 2 respectively
Answer
D
Question. The con-ect order of boiling points of the hydrides of nitrogen family is
(a) NH3 > PH3 > AsH3 > SbH3
(b) PH3 < AsH3 < NH3 < SbH3
(c) NH3 < PH3 < SbH3 < AsH3
(d) NH3 < PH3 < AsH3 < SbH3
Answer
B
Question. SO2 + H2S → Product.The final product is
(a) H2O + S
(b) H2SO4
(c) H2SO3
(d) H2SO3
Answer
A
Question. The stable bivalency ofpb and trivalency of Bi is
(a) due to d contraction in Pb and Bi
(b) due to relativistic contraction of the fu.orbitals of Pb and Bi, leading to inert pair effect
(c) due to screening effect
(d) due to attainment of noble liquid configuration
Answer
B
Question. Atomicity of sulphur in rhombic sulphur is
(a) 1
(b) 2
(c) 8
(d) 6
Question. Oxygen molecule is
(a) diamagnetic with no-unpaired electron(s)
(b) diamagnetic with two unpaired electrons
(c) paramagnetic with two unpaired electrons
(d) paramagnetic with no unpaired electron(s)
Answer
C
Question. Which of the following on thermal decomposition gives oxygen gas ?
(a) Ag2O
(b) Pb3O4
(c) PbO2
(d) All of these
Answer
D
Question. On heating KClO3 we get
(a) KClO2 + O2
(b) KCl + O2
(c) KCl + O3
(d) KCl + O2 + O3
Answer
B
Question. Which of the following is not oxidized by O3 ?
(a) KI
(b) FeSO4
(c) KMnO4
(d) K2MnO4
Answer
C
Question. About 20 km above the earth, there is an ozone layer. Which one of the following statements about ozone and ozone layer is true?
(a) Ozone has a triatomic linear molecule
(b) It is harmful as it stops useful radiation
(c) It is beneficial to us as it stops U.V radiation
(d) Conversion of O3 to O2 is an endothermic reaction
Answer
C
Question. Oxygen gas can be prepared from solid KMnO4 by :
(a) treating the solid with H2 gas
(b) strongly heating the solid
(c) dissolving the solid in dil. H2SO4
(d) dissolving solid in dil. HCl
Answer
B
Question. Which of the following statements is correct :
(a) Ozone is a resonance hybrid of oxygen
(b) Ozone is an isomer of oxygen
(c) Ozone has no relationship with oxygen
(d) Ozone is an allotropic modification of oxygen
Answer
D
Question. Which of the following is an acidic oxide?
(a) Mn2O7
(b) Na2O
(a) N2O
(b) BaO
Answer
A
Question. The compound which gives off oxygen on moderate heating is :
(a) cupric oxide
(b) mercuric oxide
(c) zinc oxide
(d) aluminium oxide
Answer
B
Question. Which of the following form of the sulphur shows paramagnetic behaviour ?
(a) S8
(b) S6
(c) S2
(d) All of these
Answer
C
Question. Number of bonds in SO2 are
(a) two σ and two π
(b) two σ and one π
(c) two σ and three π
(d) None of these
Answer
B
Question. What is X in the following reaction ?
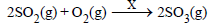
(a) V2O5
(b) CuO
(c) CuCl2
(d) MnO2
Answer
A
Question. Which of the following oxo acid of sulphur has O–O bond ?
(a) H2S2O7
(b) H2S2O8
(c) H2S2O6
(d) H2S2O5
Answer
B
Question. The number of electrons that are paired in oxygen molecule are
(a) 16
(b) 12
(c) 14
(d) 7
Answer
C
Question. Bleaching action of SO2 is due to its
(a) oxidising property
(b) acidic property
(c) reducing property
(d) basic property
Answer
C
Question. Hybridization of S in SO3 is
(a) sp2
(b) sp3
(c) sp2d
(d) sp3d2
Answer
A
Question. Carbohydrates on reaction with conc. H2SO4 becomes charred due to
(a) hydrolysis
(b) dehydration
(c) hydration
(d) oxidation
Answer
B
Question. By which of the following SO2 is formed ?
(a) Reaction of dil. H2SO4 with O2
(b) Hydrolysis of dil. H2SO4
(c) Reaction of conc. H2SO4 with Cu
(d) None of these
Answer
C
Question. Which of the following is the key step in the manufacture of sulphuric acid ?
(a) Burning of sulphur or sulphide ores in air to generate SO2
(b) Conversion of SO2 to SO3 by the reaction with oxygen in presence of catalyst.
(c) Absorption of SO3 in H2SO4 to give oleum.
(d) Both (b) and (c)
Answer
B
Question. Calcium cyanamide on treatment with steam produces
(a) NH3 + CaO
(b) NH3 + CaHCO3
(c) NH3+ CaCO3
(d) NH3 + Ca(OH)2
Answer
C

We hope you liked the above provided MCQ Questions Chapter 7 The p – Block Elements Class 12 Chemistry with solutions. If you have any questions please ask us in the comments box below.


