Please refer to MCQ Questions Chapter 11 Alcohols Phenols and Ethers Class 12 Chemistry with answers provided below. These multiple-choice questions have been developed based on the latest NCERT book for class 12 Chemistry issued for the current academic year. We have provided MCQ Questions for Class 12 Chemistry for all chapters on our website. Students should learn the objective based questions for Chapter 11 Alcohols Phenols and Ethers in Class 12 Chemistry provided below to get more marks in exams.
Chapter 11 Alcohols Phenols and Ethers MCQ Questions
Please refer to the following Chapter 11 Alcohols Phenols and Ethers MCQ Questions Class 12 Chemistry with solutions for all important topics in the chapter.
MCQ Questions Answers for Chapter 11 Alcohols Phenols and Ethers Class 12 Chemistry
Question. The correct order of boiling point for primary (1°), secondary (2°) and tertiary (3°) alcohols is
(a) 1° > 2° > 3°
(b) 3° > 2° > 1°
(c) 2° > 1° > 3°
(d) 2° > 3° > 1°
Answer
A
Question. The alcohol that produces turbidity immecliately with ZnCl2 / cone. HCl at room temperature
(a) 1-hydroxy butane
(b) 2-hydroxy butane
(c) 2-hydroxy-2-methyl propane
(d) 1-hydroxy-2-methyl propane
Answer
C
Question. Which one of the following alcohol is used as an antifreeze reagent for making explosives ?
(a) Glycerol
(b) Glycol
(c) Ethanol
(d) Phenol
Answer
A
Question. Which one can differentiate between C2H5OH and CH3OH?
(a) H2O
(b) Na2CO3 + I2
(c) NH3
(d) HCl
Answer
B
Question. An unknown compound’ D’ first oxidised to aldehyde and then acetic acid by a dilute solution of K2 Cr2O7 and H2SO4 . The compound ‘D’ is
(a) CH3OH
(b) C2H5OH
(c) CH3CH2COOH
(d) CH3CH2CHO
Answer
B
Question. Ethylene glycol reacts with excess of PCI5 to give
(a) 1, 1-dichloroethane
(b) 1, 2-clichloroethane
(c) 1, 1, 1-trichloroethane
(d) 2, 2-clichloroethane
Answer
B
Question. The correct order of reactivity of hydrogen halides with ethyl alcohol is
(a) HF > HCl > HBr > HI
(b) HCl > HBr > HF > HI
(c) HBr > HCl > HI > HF
(d) HI > HBr > HCl > HF
Answer
D
Question. Phenol on treatement with diethyl sulphate in presence of NaOH gives
(a) phenetole
(b) anisole
(c) diphenyl ether
(d) diethyl ehter
Answer
A
Question. A fruity smell is obtained by the reaction of ethanol with
(a) CH3COCH3
(b) PCI5
(c) CH3COOH
(d) CH3CHO
Answer
C
Question. Denatured alcohol is
(a) ethanol + methanol
(b) rectified spirit+ methanol + naphtha
(c) undistilled ethanol
(d) rectified spirit
Answer
A
Question. Glycerol on heating with oxalic acid at 110°C gives
(a) ethanol
(b) methanoic acid
(c) ether
(d) acetone
Answer
B
Question. Among the following compounds which can be dehydrated very easily ?

Answer
C
Question. Cyclohexanol on reaction with PBr3 in presence of pyridine gives
(a) bromocyclohexene
(b) bromocyclohexane
(c) 1-bromocyclohexanol
(d) None of these
Answer
B
Question.

Answer
C
36. Correct acidic order of the following compounds is
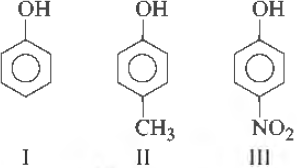
(a) I > II > III
(b) III > I > II
(c) II > III > I
(d) I > III > II
Answer
B
Question. Which of the following reagents may be used to clistinguish between phenol and benzoic acid?
(a) Aqueous NaOH
(b) Tollen’s reagent
(c) Molisch reagent
(d) Neutral FeCI3
Answer
D
Question. The correct order of acid strength of the following compounds is
I. Phenol
II p-cresol
III. m-nitrophenol
IV. p-nitrophenol
(a) III > II > I > IV
(b) IV > III > I > II
(c) II > IV > I > III
(d) I > II > IV > III
Answer
B
Question. In the reaction, A →H2SO4K2Cr2C7 acetone →Oxidation aceticacid,A is
(a) 1-propano
(b) 2-butanoll
(c) 2-propanol
(d) ethanol
Answer
C
Question,

Answer
A
Question. lodoform reaction is answered by all, except
(a) CH3—CH—CH2—COOH
l
OH
(b) CH3CHO
(c) CH3—CH2—OH
(d) CH3—CH2—CH2OH
Answer
D
Question. 2-propanol + NaBr →Reflux X. What is X ?
(a) 2-bromopropane
(b) Propane
(c) Propene
(d) Propanone
Answer
A
Question. Which of the following will not react with NaOH?
(a) C2H5
(b) C2H5OH
(c) CH3CONH2
(d) CH(CN)3
Answer
B
Question. Lucas reagent is
(a) cone. HCl and anhydrous ZnCI2
(b) cone. HNO3 and hydrous ZnCI2
(c) cone. HCl and hydrous ZnCI2
(d) cone. HNO3 and anhydrous ZnCI2
Answer
A
Question. In the following reaction, X and Y respectively are
C2H5OH →KMnO4/H+ X →H2SO4 / ΔY CH3CO2C2H5
(a) CH3OH, C2H5OH
(b) CH3CHO, CH3OH
(c) CH3CO2 H, C2H5OH
(d) C2H4 , CH3CO2H
Answer
C
Question. When phenol is treated with D2SO4 / D2O , some of the hydrogens get exchanged. The final product in this exchange reaction is
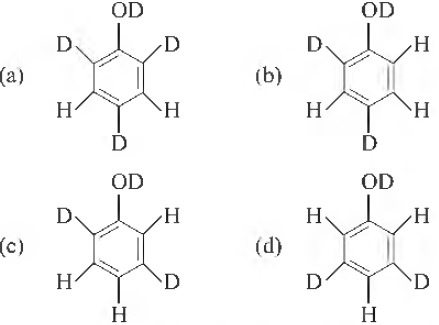
Answer
A
Question. X→Y Benzoquinone, Identify X and y in the given reaction.
Answer
B
Question. When phenol is treated with excess of bromine water, it gives
(a) m-bromophenol
(b) o-and p-bromophenol
(c) 2, 4-dibromophenol
(d) 2,4,6-tribromophenol
Answer
D
Question. CH3CH2OH →CI2step-1 CH3CHO →3CI2step-2 CI3CCHO In above reactions the role of Cl2 in step 1 and step 2 respectively is
(a) oxidation, chlorination
(b) reduction, chlorination
(c) oxidation, addition
(d) reduction, substitution
Answer
A
Question. Glycerol is oxidised by bismuth nitrate to produce
(a) oxalic acid
(b) meso oxalic acid
(c) glyceric acid
(d) glyoxylic acid
Answer
B
Question. For the identification of β-naphthol using dye test, it is necessary to use
(a) dichloromethane solution of P-naphthol
(b) acidic solution of β-naphthol
(c) neutral solution of β-naphthol
(d) alkaline solution of β-naphthol
Answer
D
Question. Sodiwn phenoxide when heated with CO2 under pressure at 125° C yields a product which on acetylation produces C.

Answer
A
Question. Which of the following compounds will give positive iodoform test with I2 and NaOH?
(a) C6H5COC6H5
(b) CH3CH2CHO
(c) C6H5COCH2CH3
(d) C6H5—CH—CH3
l
OH
Answer
D
Question. Which of the following is dihydric alcohol? [Manipall
(a) Glycerol
(b) Ethylene glycol
(c) Catechol
(d) Resorcinol
Answer
B
Question. An organic compound A containing C, H and O has a pleasant odour with boiling point of 78°C. On boiling A with concentrated H2SO4 , a colourless gas is produced which decolourises bromine water and alkaline KMnO4 • The organic liquid A is
(a) C2H5Cl
(b) C2H5COOCH3
(c) C2H5OH
(d) C2H6
Answer
C
Question. The dehydration of butan-1-ol gives
(a) 1-butene as the main product
(b) 2-butene as the main product
(c) equal amounts of 1-butene and 2-butene
(d) 2-methyl propene
Answer
B
Question. Methanol cannot be dried with anhydrous CaCl2 because
(a) CaCI2 dissolves in it
(b) it is not good dehydrating agent
(c) it forms a solid CaCI2 ·4 CH3OH
(d) it reacts with CH3OH
Answer
C
Question. In the reaction, C2H5OH →Cu300°C X The molecular formula of X is
(vapour)
(a) C4H6O
(b) C4H10O
(c) C2H4O
(d) C2H6
Answer
C
Question. The correct order of ease of dehydration of following is
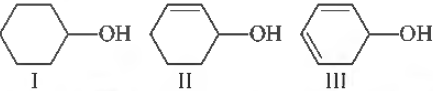
(a) I > II > III
(b) III > II > I
(c) I > III > II
(d) III > I > II
Answer
B
Question. Alcoholic beverages contain
(a) iso-propyl alcohol
(b) n-propyl alcohol
(c) ethyl alcohol
(d) methyl alcohol
Answer
C
Question. Which of the following compounds is most acidic?
(a) CH4
(b) C2H6
(c) CH=CH
(d) C2H5OH
Answer
D
Question. The reactivity of compound Z with different halogens under appropriate conditions is given below

The observed pattern of electrophilic substitution can be explained by
(a) the steric effect on the halogen
(b) the steric effect of the tert-butyl group
(c) the electronic effect of the phenolic group
(d) the electronic effect of the tert-buty 1 group
Answer
A.B.C
Question. Identify the correct product formed during the following reaction
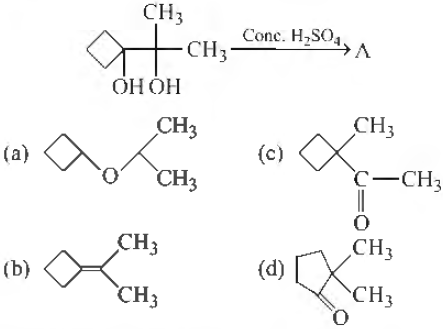
Answer
D
Question. What represents the best method producing salicylic acid?
(a) Benzoic acid, strong base, high temperature
(b) Toluene, strong base, water, high temperature
(c) Phenol, base, carbon dioxide then acid
(d) Bromobenzene, carbonic acid, high temperature
Answer
C
Question.

In the above sequence, C is
(a) o-bromophenol
(b) p-bromophenol
(c) m-bromophenol
(d) 2, 4, 6-tribromophenol
Answer
D
Question. The major product(s) of the following reaction is (are)

(a) P
(b) Q
(c) R
(d) S
Answer
B
Question. What reaction does hydroquinone undergo most readily?
(a) SN2substitution
(b) Hydrolysis
(c) Oxidation
(d) Reduction
Answer
C
Question. In the following reaction, the product(s) formed is (are)
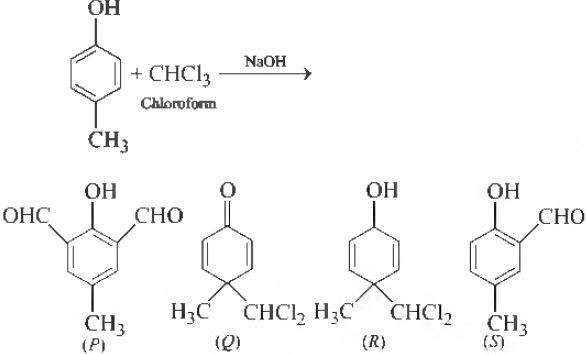
(a) P (major)
(b) Q (minor)
(c) R (minor)
(d) S (major)
Answer
B.D
Question. Phenol is heated with a solution of mixture of KBr and KBrO3 . The major product obtained in the above reaction is
(a) 2-bromophenol
(c) 4-bromophenol
(b) 3-bromophenol
(d) 2, 4, 6-tribromophenol
Answer
D
Question. The conversion of m-nitrophenol to resorcinol involves respectively
(a) hydrolysis, cliazotisation and reduction
(b) diazotisation, reduction and hydrolysis
(c) hydrolysis, reduction and cliazotisation
(d) reduction, cliazotisation and hydrolysis
Answer
D
Question. Phenol can be converted too-hydroxy benzaldehyde by
(a) Kolbe’s reaction
(b) Reimer-Tiemann reaction
(c) Wurtz reaction
(d) Cannizaro reaction
(e) Sandmeyer’s reaction
Answer
B
Question. Two aromatic compounds having formula C7H8O which are easily identifiable by FeCI3 solution test (violet colouration) are
(a) o-cresol and benzyl alcohol
(b) m-cresol and p-cresol
(c) o-cresol and p-cresol
(d) methyl phenyl ether and benzyl alcohol
Answer
A
Question. The correct order of decreasing acidity of nitrophenols will be
(a) m-nitrophenol > p-nitrophenol > o-nitrophenol
(b) o-nitrophenol > m-nitrophenol > p -nitrophenol
(c) p -nitrophenol > m-nitrophenol > o-nitrophenol
(d) p-nitrophenol > o-nitrophenol > m-nitrophenol
Answer
D
Question. Phenol →X forms a tribromo derivative “X” is
(a) bromine in benzene
(b) bromine in water
(c) potassium bromide solution
(d) bromine in carbon tetrachloride at 0°C
Answer
B
Question. The major product obtained on interaction of phenol with sodium hydroxide and carbon dioxide is
(a) benzoic acid
(b) salicylaldehyde
(c) salicylic acid
(d) phthalic acid
Answer
C
Question. The order of melting point ofortho, para, meta nitrophenol is
(a) o > m > p
(b) p > m > o
(c) m > p > o
(d) p > o > m
Answer
B
Question. Consider the following reaction,
C2H5OH + H2SO4 → Product
Among the following, which one cannot be formed as a product under any conditions?
(a) Ethyl hydrogen sulphate
(b) Ethylene
(c) Acetylene
(d) Diethyl ether
Answer
C
Question. During dehydration of alcohols to alkenes by heating with concentrated H2SO4 the initiation step is
(a) protonation of alcohol molecule
(b) formation of carbocation
(c) elimination of water
(d) formation of an ester
Answer
A
Question. Chlorobenzene →ReactionX Phenol →ReactionY
Salicylaldehyde
X and Y reactions are respectively ….. .
(a) Fries rearrangement and Kolbe-Schmidt
(b) Cumene and Reimer-Tiemann
(c) Dow and Reimer-Tiemann
(d) Dow and Friedel-Craft
Answer
C
Question. Acidity of phenol is due to
(a) hydrogen bonding
(b) phenolic group
(c) benzene ring
(d) resonance stabilisation of its anion
Answer
D
Question. In the reaction for dinitration

The major dinitrated productXis

Answer
A
Question. Phenol, when it first reacts with concentrated sulphuric acid and then with concentrated nitric acid, gives
(a) 2, 4, 6-trinitrobenzene
(b) o-nitrophenol
(c) p -nitrophenol
(d) nitrobenzene
Answer
B
Question. An organic acid without a carboxylic acid group is
(a) picric acid
(b) oxalic acid
(c) vinegar
(d) ascorbic acid
Answer
A
Question. When ethyl alcohol is heated with cone. H2SO4 , the product obtained is
(a) CH3COOC2H5
(b) C2H2
(c) C2H6
(d) C2H4
Answer
D

We hope you liked the above provided MCQ Questions Chapter 11 Alcohols Phenols and Ethers Class 12 Chemistry with solutions. If you have any questions please ask us in the comments box below.


