Please refer to Hydrocarbons Class 11 Chemistry Important Questions with solutions provided below. These questions and answers have been provided for Class 11 Chemistry based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these problem solutions as it will help them to gain more marks in examinations. We have provided Important Questions for Class 11 Chemistry for all chapters in your book. These Board exam questions have been designed by expert teachers of Standard 11.
Class 11 Chemistry Important Questions Hydrocarbons
Very Short Answer Type Questions :
Question. Explain why the branching of an alkane chain lowers its boiling point.
Question Boiling point decreases with increase in branching due to decrease in surface area of the molecule.
Question. Name the chain isomer of C5H12 which hasa tertiary hydrogen atom.
Answer : 2-Methylbutane, (CH3)2CH — CH2 — CH3.
Question. Explain why dry ether is used as a solvent in Wurtz reaction.
Answer : In Wurtz reaction, pure sodium is used which is highly violent towards water therefore, dry ether is used.
Question. When alkyne is treated with bromine water then what will be the colour of product?
Answer : The product will be colourless.
Question. Complete the following reaction :
CH3 — CH == CH2 + HBr Organic peroxide
Answer :
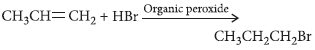
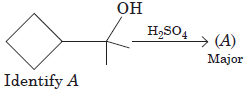
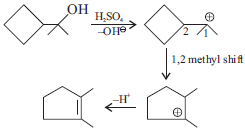
Question. What product would you get from acid catalysed hydration of 1-methylcyclohexene? Explain.
Answer : 1-Methylcyclohexanol will be formed because a 3° carbocation will be formed as an intermediate.

Question. Acetylene is acidic but it does not react with NaOH or KOH. Give reason.
Answer : Due to sp-hybridisation of C-atom in acetylene, proton is strongly attracted by nucleus and cannot be abstracted easily therefore, it does not react with NaOH or KOH.
Question. Draw the Newmann projection formula forstaggered and eclipsed conformation of ethane.
Answer :

Question. Give a brief account for the following statement :
CH4 cannot be synthesized by Wurtz reaction.
Answer : Wurtz reaction occurs between two alkyl halides to yield alkane. Methane has only one carbon atom, hence cannot be prepared by using Wurtz reaction.
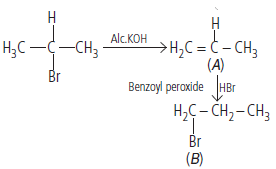
Question. Write structures of A and B in the following reaction :
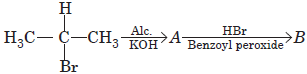
Answer :
Short Answer Type Questions :
Question. Which alkyne would you start with and what reagents would you use to prepare :
(i) cis-but-2-ene
(ii) trans-pent-2-ene
Answer :


Question. What does ozonolysis of benzene yield?
Answer :
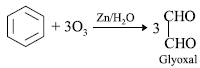
Question.
Answer :
Question. What do the following compounds produce when passed through Cr2O3 supported over aluminia at 600°C?
(i) n-Hexane
(ii) n-Heptane
Answer :

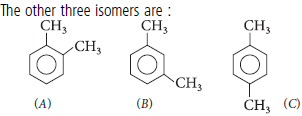
Question. A hydrocarbon (Z) has molecular formula C8H10. It does not decolourise bromine water and is oxidised to benzoic acid on heating with K2Cr2O7. It can also have three other isomers A, B and C. Write the structures of Z, A, B and C.
Answer : Since, it does not decolourise bromine water, it is arene. Thus,
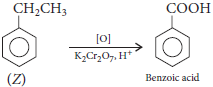
Question. An alkyne (X) has molecular formula C5H8. It reacts neither with sodamide nor with ammoniacal cuprous chloride. Identify X.
Answer : Alkyne X is C5H8. Since it does not react with sodamide or ammoniacal cuprous chloride, the triple bond cannot be terminal.
∴ X is CH3CH2C ≡ CCH3 (Pent-2-yne)
Question.

Answer :
Question. Ethyne reacts with dil. H2SO4 in presence of mercury salt to give acetaldehyde but with dil. HCl under similar conditions, it gives vinyl chloride. Explain why.
Answer : Mercuric ion forms a complex (I) with acetylene. Since, H2O is more nucleophilic than SO42− ion, it attacks the complex
(I) to form first vinyl alcohol which further tautomerises to give acetaldehyde.

Question. Why cis-but-2-ene has higher boiling point than trans-but-2-ene?
Answer : Due to higher dipole moment, the boiling point of cisisomer is higher than the corresponding trans-isomer.
Question. How will you convert methyl bromide to ethane?
Answer : Two moles of methyl bromide react with sodium metal in presence of dry ether as solvent to give ethane. This reaction is known as Wurtz reaction.

Question.

Identify X and Y .Answer : On heating with alc. KOH in inert solvent, the triple bond of 1-alkyne is shifted towards the centre to form an isomeric 2-alkyne. On heating with sodamide (NaNH2 in liq. NH3) the triple bond shifts towards end.
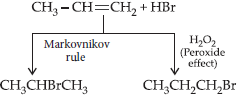
Question. Explain anti-Markovnikov addition or peroxide effect or Kharash effect with example.
Answer : Peroxide effect : Addition of HBr in presence of peroxide gives products opposite to Markovnikov rule.
Question. Why is Wurtz reaction not preferred for the preparation of alkanes containing odd number of carbon atoms?
Answer : It is because mixture of alkanes will be formed e.g.,
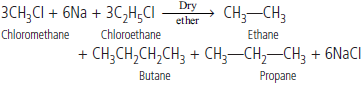
Question. (a) Write chemical reactions to illustrate the Kolbe’s reaction
(b) Name the compound that will be required to obtain butane using Kolbe’s electrolysis process.
Answer : (a) Kolbe’s reaction : In this reaction, an aqueous solution of sodium or potassium salt of carboxylic acid on electrolysis gives alkane having even number of carbon atoms at anode.
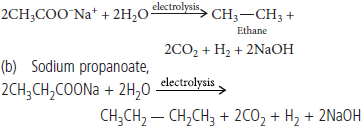
Question. Give two reactions to show acidic character of alkynes.
Answer :

Question. Draw the Newman projections of the eclipsed and staggered conformers of ethane. Which of the two is stable and why?
Answer :

In staggered form of ethane, the electron clouds of carbonhydrogen bonds are as far apart as possible. Thus, there are minimum repulsive forces, minimum energy and maximum stability of the molecule. On the other hand, when the staggered form changes into the eclipsed form, the electron clouds of the carbon-hydrogen bonds come closer to each other resulting in increase in electron cloud repulsions. Thus, the molecule has more energy and therefore, has lesser stability.
Question. (a) Explain the order of stability of carbocations giving reason.
(b) Addition of HBr to propene in the presence of benzoyl peroxide yields 1-bromopropane. Explain with suitable mechanism.
Answer : (a) Stability of carbocations decreases as 3° > 2° > 1°. Alkyl groups have +I effect. when an alkyl group is attached to positively charged carbon atom of a carbocation, it tends to release electrons towards that carbon and reduces the positive charge on the carbon. Thus, positive charge gets dispersed. This dispersal of the positive charge stabilises the carbocation.
(b) Mechanism : Peroxide effect proceeds via free radical mechanism as given below :
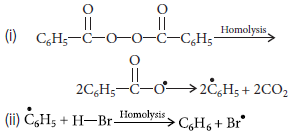

Question. Explain Friedel–Crafts alkylation reaction with chemical equation.
Answer : Friedel–Crafts alkylation is a Lewis acid-catalyzed electrophilic aromatic substitution reaction that allows the synthesis of alkylated products via the reaction of arenes with alkyl halides. With anhydrous aluminium chloride as a catalyst, the stable alkyl carbocation is generated which attacks the benzene ring. An example of this type of reaction is shown below :
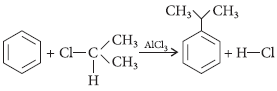
Question. An alkene ‘A’ contains three C — C, eight C — H σ bonds and one C — C π bond. ‘A’ on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of ‘A’.
Answer : Alkene A contains 3C — C, 8C — H and one C = C bonds. An aldehyde containing one —CHO group and having molar mass of 44 amu has to be CH3CHO and since two moles of CH3CHO are obtained by ozonolysis of alkene A, the alkene has to be joined by two CH3CH— groups by a double bond. It has to be CH3 — CH CH — CH3, i.e., but-2-ene. But-2-ene contains 3C—C σ bonds, 8C — H σ bonds and one C C bond.

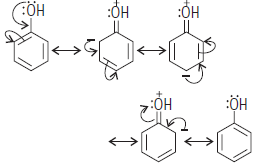
Question. Explain ortho- and para-directing influence of monosubstituted benzene giving suitable example.
Answer : —OH group attached to benzene ring release electrons and activate the benzene ring, direct the incoming groups to ortho and para positions.
Question. Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also, give reason for this behaviour.
Answer :

Since s-orbitals are closer to the nucleus, hence due to more s-character in ethyne (sp hybridised), the hybridised orbital is nearest to this carbon atom in comparison to sp3 or sp2 hybridised carbon. This leads to the movement of C — H bond pair more towards sp hybridised carbon, leading to the development of partial positive charge on the hydrogen attached to sp hybridised carbon. Thus, such a hydrogen behaves as acidic hydrogen. Hence, order of acidic nature is, ethyne > benzene > n-hexane.
Question. An organic compound A with molecular formula C3H8O reacts with conc. H2SO4 to give B, which on reaction with HCl gives C. Compound C reacts with metallic sodium to give D. Identify compounds A, B, C and D.
Answer : Compound A is an alcohol which on reaction with H2SO4 gives alkene B.
Question. Identify a reagent which can easily distinguish between 1-butyne and 2-butyne.
Answer : There will be no reaction between 2-butyne and Cu2Cl2 because it has no acidic hydrogen. In 1-butyne the terminal hydrogen is acidic (CH3CH2 – C ≡ CH) so it will give a red ppt. with ammoniacal Cu2Cl2.
Question. Complete the following reactions :
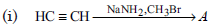

Answer :

Question. Despite their –I effect, halogens are o- and p-directing in haloarenes. Explain.
Answer : In case of aryl halides, halogens are little deactivating because of their strong –I-effect. Therefore, overall electron density on the benzene ring decreases. In other words, halogens are deactivating due to –I-effect. However, because of the +R-effect, i.e., participation of lone pairs of electrons on the halogen atom with the p-electrons of the benzene ring, the electron density increases more at o- and p-positions than at m-positions.
As a result, halogens are o, p-directing. The combined result of +R-effect and –I-effect of halogens is that, halogens are deactivating but o, p-directing.
Long Answer Type Questions :
Question. Explain why the following systems are not aromatic.

Answer : (a)

it has 6p-electrons but not in the ring and one carbon atom has sp3-hybridisation, hence it is nonaromatic.
(b) In
due to the presence of sp3-hybridised carbon (carbon 3) and only four p electrons, it does not contain planar delocalised cloud of (4n + 2)π electrons. Hence, it is nonaromatic compound.
(c) Cyclooctatetraene (COT) is not aromatic because of its non-planar tub-shaped structure. Athough according to
electron-count it seems to be an anti-aromatic compound but,

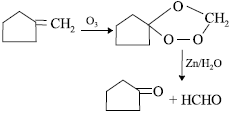
Question. A hydrocarbon ‘Y’ decolourises bromine water. On ozonolysis it gives 3-methylbutanal and formaldehyde. Give the name of the compound. Identify Y.
Answer : Hydrocarbon ‘Y ’ is alkene because it decolourises bromine water. From the products of ozonolysis, the structure of alkene can be predicted.

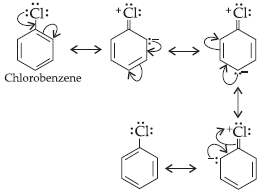
Question. Give mechanism for the following reaction.
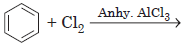
Answer : The halogenation of benzene proceeds by the following mechanism :

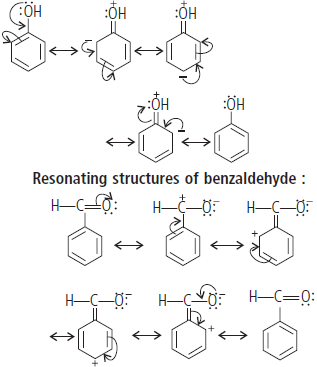
Question. Draw the resonating structure of C6H5OH (phenol) and C6H5CHO (benzaldehyde).
Answer : Resonating structures of phenol :
—OH group attached to benzene ring release electrons and activate the benzene ring, direct the incoming groups to ortho- and para-positions.
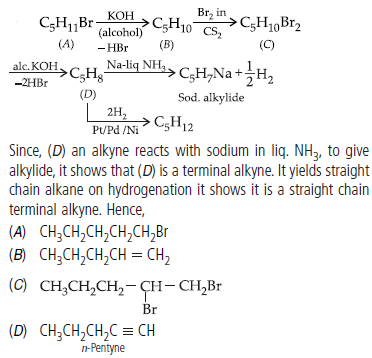
Question. An alkyl halide C5H11Br (A) reacts with ethanolic KOH to give an alkene ‘B’, which reacts with Br2 to give a compound ‘C’, which on dehydrobromination gives an alkyne ‘D’. On treatment with sodium metal in liquid ammonia one mole of ‘D’ gives one mole of the sodium alt of ‘D’ and half a mole of hydrogen gas. Complete hydrogenation of ‘D’ yields a straight chain alkane. Identify A,B,C and D. Give the reactions involved.
Answer :