Please refer to Assignments Class 12 Chemistry Electrochemistry Chapter 3 with solved questions and answers. We have provided Class 12 Chemistry Assignments for all chapters on our website. These problems and solutions for Chapter 3 Electrochemistry Class 12 Chemistry have been prepared as per the latest syllabus and books issued for the current academic year. Learn these solved important questions to get more marks in your class tests and examinations.
Electrochemistry Assignments Class 12 Chemistry
Question. When 0.1 mol CoCl3(NH3)5 is treated with excess of AgNO3, 0.2 mol of AgCl are obtained. The conductivity of solution will correspond to:
(A) 1: 3 electrolyte
(B) 1: 2 electrolyte
(C) 1: 1 electrolyte
(D) 3: 1 electrolyte
Answer
B
Question. Which of the following statements is not correct ?
(A) Copper liberates hydrogen from acids.
(B) In its higher oxidation states, manganese forms stable compounds with oxygen and fluorine.
(C) Mn3+ and Co3+ are oxidising agents in aqueous solution.
(D) Ti2+ and Cr2+ are reducing agents in aqueous solution.
Answer
A
Question. Which of the statements about solutions of electrolytes is not correct ?
(A) Conductivity of solution depends upon size of ions.
(B) Conductivity depends upon viscosity of solution.
(C) Conductivity does not depend upon solvation of ions present in solution.
(D) Conductivity of solution increases with temperature.
Answer
C
Question. Debye-Huckel Onsager equation for strong electrolytes:

Answer
C
Question. The cell constant of a conductivity cell __________.
(A) Changes with change of electrolyte.
(B) Changes with change of concentration of electrolyte.
(C) Changes with temperature of electrolyte.
(D) Remains constant for a cell.
Answer
D
Question. In the electrolysis of aqueous sodium chloride solution which of the half cell reaction will occur at anode?

Answer
D
Question. What will happen during the electrolysis of aqueous solution of CuSO4 in the presence of Cu electrodes?
(A) Copper will deposit at cathode
(B) Copper will dissolve at anode
(C) Oxygen will be released at anode
(D) Copper will deposit at anode
Answer
A,B
Question. Which of the following statement is correct?
(A) ECell and ΔrG of cell reaction both extensive properties.
(B) ECelland ΔrG of cell reaction both intensive properties.
(C) ECell is an intensive property while ΔrG of cell reaction is an extensive property.
(D) ECell is an extensive property while ΔrG of cell reaction is an intensive property.
Answer
C
Question. Reduction potentials of some ions are given below. Arrange them in decreasing order of oxidising power.
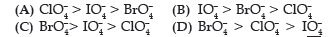
(A) ClO4– > IO4– > BrO4–
(B) IO4– > BrO4– > ClO4–
(C) BrO4–> IO4– > ClO4–
(D) BrO4– > ClO4– > IO4–
Answer
C
Question. An electrochemical cell behaves like an electrolytic cell when:
(A) ECell = Eexternal
(B) ECell = 0
(C) Eexternal > ECell
(D) Eexternal < ECell
Answer
C
Question. Which of the following option will be the limiting molar conductivity of CH3COOH if the limiting molar conductivity of CH3COONa is 91 Scm2mol–1? Limiting molar conductivity for individual ions are given in the following table.
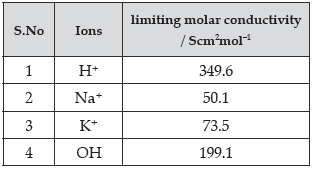
(A) 350 Scm2mol–1
(B) 375.3 Scm2mol–1
(C) 390.5 Scm2mol–1
(D) 340.4 Scm2mol–1
Answer
C
Question. Electrode potential for Mg electrode varies according to the equation:
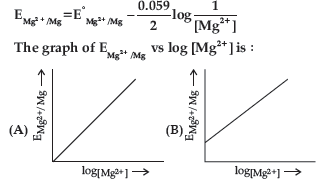
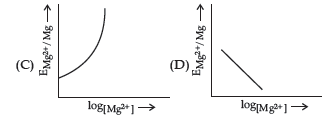
Answer
B
Question. In an electrochemical process, a salt bridge is used:
(A) as a reducing agent
(B) as an oxidizing agent
(C) to complete the circuit so that current can flow
(D) None of these
Answer
C
Question. Consider the following reaction:
Cu(s) + 2Ag+(aq) → 2Ag(s) + Cu2+(aq)
Depict the galvanic cell in which the given reaction takes place.
(A) Cu2+ (aq)|Cu(s) ||Ag+(aq)|Ag(s)
(B) Cu(s) | Cu2+(aq) || Ag+ (aq) | Ag(s)
(C) Ag+(aq)|Ag(s)||Cu2+ (aq)|Cu(s)
(D) Ag(s)|Ag+(aq)||Cu2+ (aq)|Cu(s)
Answer
B
Question. Conductivity k, is equal to ______________.

Answer
B
Question. A°m [NH4 OH] is equal to ______ .
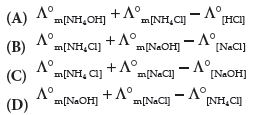
Answer
B
Question. Calculate the emf of the following cell at 298 K:
Mg(s)|Mg2+ (0.1 M)||Cu2+ (1.0 × 10–3 M)|Cu(s)
[Given = E°Cell = 2.71 V]
(A) 1.426 V
(B) 2.503 V
(C) 2.651 V
(D) 1.8 V U
Answer
C
Question. Using the data given below find strongest reduction agent.
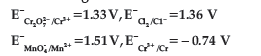
(A) Cl-
(B) Cr
(C) Cr3+
(D) Mn2+
Answer
B
Question. Following reactions occur at cathode during the electrolysis of aqueous silver chloride solution:
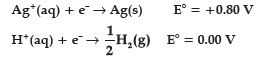
On the basis of their standard reduction electrode potential (E°) values, which reaction is feasible at the cathode ?

(C) Both reactions are feasible
(D) None of these above
Answer
A
Question. What will happen during the electrolysis of aqueous solution of CuSO4 by using platinum electrodes?
(A) Copper will deposit at cathode.
(B) Copper will deposit at anode.
(C) Oxygen will be released at anode.
(D) Copper will dissolve at anode.
Answer
A,C
Assertion and Reason Based MCQs
Directions: In the following questions, a statement of
Assertion (A) is followed by a statement of Reason (R).
Mark the correct choice as:
(A) Both (A) and (R) are true, and (R) is the correct explanation of (A).
(B) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(C) (A) is true, but (R) is false.
(D) (A) is false, but (R) is true.
Question. Assertion (A): Ecell should have a positive value for the cell to function.
Reason (R): Ecathode < Eanode
Answer
C
Question. Assertion (A): Electrolytic conduction increases with increase in temperature.
Reason (R): Increase in temperature cause the electronic movement more rapid.
Answer
C
Question. Assertion (A): Copper sulphate can be stored in zinc vessel.
Reason (R): Zinc is more reactive than copper.
Answer
D
Question. Assertion (A): Molar conductivity of an ionic solution depends on temperature.
Reason (R): Molar conductivity of an ionic solution depends on the concentration of electrolytes in the solution.
Answer
B
Question. Assertion (A): Conductivity of an electrolyte increases with decrease in concentration.
Reason (R): Number of ions per unit volume decreases on dilution.
Answer
D
Question. Assertion (A): Cu is less reactive than hydrogen.
Reason (R): E ° Cu2+/Cu is negative.
Answer
C
Question. Assertion (A): Electrolysis of NaCl solution gives chlorine at anode instead of O2.
Reason (R): Formation of oxygen at anode requires over voltage.
Answer
A
Question. Assertion (A): Λm for weak electrolytes shows a sharp increase when the electrolytic solution is diluted.
Reason (R): For weak electrolytes degree of dissociation increases with dilution of solution.
Answer
A
Question. Assertion (A): EAg+/Ag increases with increase in concentration of Ag+ ions.
Reason (R): EAg+/Agg has a positive value.
Answer
B
Case-based MCQs
I. Read the passage given below and answer the following questions:
The cell constant is usually determined by measuring the resistance of the cell containing a solution whose conductivity is already known. For this purpose, we generally use KCl solutions whose conductivity is known accurately at various concentrations and at different temperatures. Consider the resistance of a conductivity cell filled with 0.1 M KCl solution is 200 W. If the resistance of the same cell when filled with 0.02 M KCl solution is 420 W.
(Conductivity of 0.1 M KCl solution is 1.29 S m–1.)
The following questions are multiple choice questions. Choose the most appropriate answer:
Question. What will happen to the conductivity of the cell with the dilution ?
(A) First decreases then increases
(B) Increases
(C) First increases then decreases
(D) Decreases
Answer
D
Question. What is the conductivity of 0.02 M KCl solution ?
(A) 0.452 S m–1
(B) 0.215 S m–1
(C) 0.614 S m–1
(D) 0.433 S m–1
Answer
C
Question. The cell constant of a conductivity cell ________.
(A) Changes with change of electrolyte.
(B) Changes with change of concentration of electrolyte.
(C) Changes with temperature of electrolyte.
(D) Remains constant for a cell.
Answer
D
Question. Which of the following is not true?
The conductivity of solutions of different electrolytes in the same solvent and at a given temperature differs due to:
(A) size of the ions in which they dissociate
(B) concentration of ions
(C) charge of the ions in which they dissociate
(D) is independent of ions movement under a potential gradient
Answer
D
Question. SI unit for conductivity of a solution is:
(A) S m–1
(B) S m2 mol–1
(C) mol cm–3
(D) S cm2 mol–1
Answer
A
II. Read the passage given below and answer the following questions:
A galvanic cell consists of a metallic zinc plate immersed in 0.1 M Zn(NO3)2 solution and metallic plate of lead in 0.02 M Pb(NO3)2 solution.
The following questions are multiple choice questions. Choose the most appropriate answer:
Question. Calculate the emf of the cell.
(A) 6.01 V
(B) 0.412 V
(C) 0.609 V
(D) 4.12 V
Answer
C
Question. Which of the following statement is not correct about an inert electrode in a cell ?
(A) It does not participate in the cell reaction.
(B) It provides surface either for oxidation or for reduction reaction.
(C) It provides surface for conduction of electrons.
(D) It provides surface for redox reaction.
Answer
D
Question. How will the cell be represented ?
(A) Zn(s)| Zn2+(aq)|| Pb2+(aq)|Pb(s)
(B) Zn2+(s)| Zn(aq)|| Pb2+(aq)|Pb(s)
(C) Pb2+(aq)|Pb(s)||Zn2+(s)| Zn(aq)
(D) Pb(s)|Pb2+(aq)||Zn2+(s)| Zn(aq)
Answer
A
Question. What product is obtained at cathode ?
(A) Zn
(B) Pb
(C) Zn2+
(D) Pb2+
Answer
B
III. Read the passage given below and answer the following questions:
Products of electrolysis depend on the nature of material being electrolysed and the type of electrodes being used. If the electrode is inert (e.g., platinum or gold), it does not participate in the chemical reaction and acts only as source or sink for electrons. On the other hand, if the electrode is reactive, it participates in the electrode reaction. Thus, the products of electrolysis may be different for reactive and inert electrodes.
Aqueous copper sulphate solution and aqueous silver nitrate solution are electrolysed by 1 ampere current for 10 minutes in separate electrolytic cells. In these questions, a statement of assertion followed by a statement of reason.
Choose the correct answer out of the following choices:
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(B) Assertion and reason both are correct statements but reason is NOT correct explanation for assertion.
(C) Assertion is correct statement but reason is wrong statement.
(D) Assertion is wrong statement but reason is correct statement.
Question. Assertion (A): Zinc sulphate cannot be stored in copper vessel.
Reason (R): Zinc is more reactive than copper.
Answer
D
Question. Assertion (A): At equilibrium condition Ecell = 0 or ΔrG = 0.
Reason (R): Ecell is zero when both electrodes of the cell are of the same metal.
Answer
B
Question. Assertion (A): The negative sign in the expression EZn2+/Zn = – 0.76V means Zn2+ cannot be oxidised to Zn.
Reason (R): Zn is more reactive than hydrogen and Zn will be oxidised and H+ will get reduced.
Answer
A
Question. Assertion (A): The mass of copper and silver, deposited on the cathode are same.
Reason (R): Copper and silver have different equivalent masses.
Answer
D
Question. Assertion (A): In a galvanic cell, chemical energy is converted into electrical energy.
Reason (R): Redox reactions provide the chemical energy to the cell.
Answer
A
Question. Solutions of two electrolytes ‘A’ and ‘B’ are diluted. The limiting molar conductivity of ‘B’ increases 1.5 times while that of ‘A’ increases 25 times. Which of the two is a strong electrolyte ?Justify your answer.
Answer. ‘B’ is a strong electrolyte.
B is a strong electrolyte which is completely dissociated into ions, but on dilution interionic forces overcome and ions are free to move. So there is slight increase in molar conductivity on dilution.
Question. Write the name of the cell which is generally used in hearing aids. Write the reactions taking place at the anode and the cathode of this cell.
Answer. Mercury cell
Anode: Zn(Hg) + 2OH– → ZnO(s) + H2O + 2e–
Cathode: HgO + H2O + 2e– → Hg(l) + 2OH–
Question. The resistivity of a 0.8M solution of electrolyte is 5 × 10–3 Ωcm. Calculate its molar conductivity.
Answer.
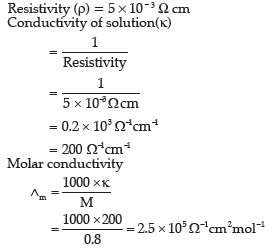
Question. In a galvanic cell, the following cell reaction occurs:
Zn(s) + 2Ag+(aq) → Zn2+(aq) + 2Ag(s)
Eocell = +1.56 V
(i) Is the direction of flow of electrons from zinc to silver or silver to zinc?
(ii) How will concentration of Zn2+ ions and Ag+ ions be affected when the cell functions?
Answer. (i) Zinc to silver
(ii) Concentration of Zn2+ ions will increase and Ag+ ions will decrease.
Question. Calculate the emf of the following cell at 298 K
Cr(s)|Cr3+ (0.1M)||Fe2+ (0.01M)|Fe(s)
[Given: E°cell = + 0.30 V]
Answer.
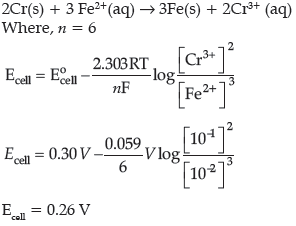
Question. From the given cells:
Lead storage cell, Mercury cell, Fuel cell and Dry cell.
Answer the following:
(i) Which cell is used in hearing aids?
(ii) Which cell was used in Apollo Space Programme?
(iii) Which cell is used in automobiles and inverters?
(iv) Which cell does not have long life?
Answer. (i) Mercury cell (ii) Fuel cell
(iii) Lead storage cell (iv) Dry cell
Question. Explain redox potential.
Reduction potentials of some ions are given below. Arrange them in decreasing order of oxidising power.

Answer. Redox potential (also known as reduction / oxidation potential) is a measure of the tendency of a chemical species to acquire electrons from or lose electrons to an electrode and thereby be reduced or oxidised respectively. Redox potential is measured in volts (V), or millivolts (mV). The more positive the reduction potential of a species, the greater the species’ affinity for electrons and tendency to be reduced.
The higher the reduction potential, the higher is its tendency to get reduced. Hence, the order of oxidising power is:
BrO4–> IO4– > ClO4–
Question. Write the electrode reactions for a H2–O2 fuel cell.
Answer.
Question. Following reactions can occur at cathode during the electrolysis of aqueous silver nitrate solution using Pt electrodes:
Ag+(aq) + e– → Ag (s); E° = 0.80 V
H+ (aq)+ e– 1/2 H2 (g) ; E° = 0.00 V
On the basis of their standard electrode potential values, which reaction is feasible at cathode and why ?
Answer. Ag+(aq) + e− → Ag(s)
Because it has higher reduction potential.
Detailed Answer: As reaction with higher value of standard electrode potential occurs at cathode, Ag gets reduced. So, the reaction occurring at cathode is
Ag+(aq) + e− → Ag(s)
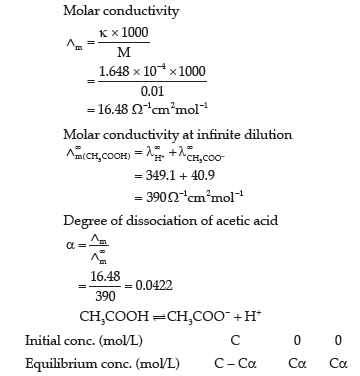
Question. Why on dilution the Lm of CH3COOH increases drastically, while that of CH3COONa increases gradually?
Answer. In case of CH3COOH which is a weak electrolyte, the number of ions increases on dilution due to an increase in degree of dissociation resulting in drastic increase in Λm.
CH3COOH+H2O → CH3COO– +H3O +
In the case of CH3COONa which is a strong electrolyte, the number of ions remains the same but the inter-ionic attraction decreases resulting in gradual increase in Λm.
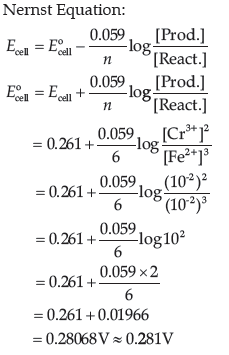
Question. Calculate E°cell for the following reaction at 298 K:
2Cr(s) + 3Fe2+ (0.01M) → 2Cr3+(0.01M) + 3Fe(s)
Given: Ecell = 0.261 V
Answer.
Question. The conductivity of 10-3 mol/L acetic acid at 25°C is 4.1 × 10–5 S cm–1. Calculate its degree of dissociation if L°m for acetic acid at 25°C is 390.5 S cm2 mol-1.
Answer.
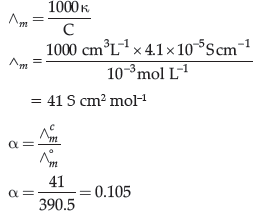
Question. Write the name of the cell which is generally used in transistors. Write the reactions taking place at the anode and the cathode of this cell.
Answer. Dry cell/Leclanche cell
Anode: Zn(s) → Zn2+ + 2e–
Cathode: MnO2 + NH4 + + e– → MnO(OH) + NH3
At cathode: MnO2 + NH+4 + e– → MnO(OH) + NH3
Question. Iron displaces copper from copper sulphate solution but Pt does not why?
Answer. Electrode potential of Fe is more than electrode potential of Cu. So, Fe displaces Cu from copper sulphate while electrode potential of Pt is less than Cu. Due to this reason, Pt cannot displace Cu from copper sulphate.
Question. Write cell representation of following cell ?
Zn(s)+2Ag+(aq) →Zn2+(aq)+2Ag(s)
Answer. Zn(s)∣Zn(aq)2+∣∣Ag(aq)+∣Ag(s)
Question. What is role of Salt bridge ?
Answer. 1. It completes internal circuit of cell. 2. It maintains electrical neutrality.
Question. What is effect of dilution on molar conductivity?
A . Molar conductivity depends on number of ions .so it increases with dilution.
Question. What is standard Hydrogen Electrode ?
Answer. It is reference electrode in which a piece of platinum coated with platinum black is dipped in 1 M HCl solution and hydrogen gas is passed at 1 atm .
Question. What is unit of Molar conductivity ?
Answer. scm2mol-1
Question. What is effect of dilution on conductivity ?
Answer. Conductivity depends on number of ions per unit volume so it decreases with dilution.
Question.The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 S cm–1. Calculate its molar conductivity?
Answer.Given, κ = 0.0248 S cm−1 c = 0.20 M molar conductivity = conductivity x1000/molarity = 124 Scm2mol-1
Question. The standard emf of Daniell cell is 1.1 V . Calculate the standard Gibbs energy for the cell reaction ?
Zn + Cu2+ → Zn2+ + Cu
Ans. ΔG° = -nFE°cell = 2x 96500 x1.1 = -212300 J/mole =-212.3 KJ/mole
Question. Calculate the emf of the cell in which the following reaction takes place:
Ni(s) + 2Ag+ (0.002 M) → Ni2+ (0.160 M) + 2Ag(s). Given that Eøcell = 1.05 V.
Answer. By using Nernst equation

= 1.05 – 0.02955 log 4 × 104
= 1.05 – 0.02955 (log 10000 + log 4)
= 1.05 – 0.02955 (4 + 0.6021)
= 0.914 V
Question. Limiting molar conductivity for NaCl, HCl and NaAc are 126.4, 425.9 and 91.0 Scm2mol–1 respectively, calculate limiting molar conductivity for Acetic acid ?
Ans. 390.5 Scm2/mol
Short Answer Type Questions-II
Question. (a) Calculate the mass of Ag deposited at cathode when a current of 2 amperes was passed through a solution of AgNO3 for 15 minutes.
(Given: Molar mass of Ag = 108 g mol–1,
1 F = 96500 C mol–1)
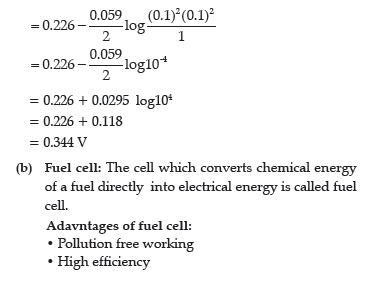
(b) Define fuel cell.
Answer.(a) t = 900 s
Charge = Current × Time = 2 × 900 = 1800 C
According to the reaction
Ag+ (aq) +e– → Ag(s)
We require 1 F to deposit 1 mol or 108 g of Ag
For 1800 C, the mass of Ag deposited will be
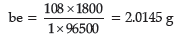
(b) Fuel cell is the name given to the galvanic cells which are designed to convert the energy of combustion of fuels like hydrogen, methane, methanol, etc. directly into electrical energy.
Question. The electrolysis of a metal salt solution was carried out by passing a current of 4 A for 45 minutes.
It resulted in deposition of 2.977 g of a metal. If atomic mass of the metal in 106.4 g mol–1, calculate the charge on the metal cation.
Answer. Let the charge on the metal ion = n+
Reduction half-reaction,
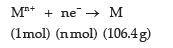
Quantity of electricity required for depositing
106.4 g of metal = n × 96500 C
Quantity of electricity required for depositing
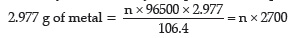
Quantity of electricity passed
= 4 × 45 × 60 C
= 10800 C
Applying law of conservation of charge,
10800 = n × 2700
n=10800/2700 = 4
Charge on metal ion = +4
Question. The electrical resistance of a column of 0.05 M KOH solution of diameter 1 cm and length 45.5 cm is 4.55 × 103 ohm. Calculate its molar conductivity.
Answer. A = Πr2
= 3.14 × 0.5 × 0.5 cm2
= 0.785 cm2
l = 45.5 cm
G* = l/A = 45.5 cm/0.785 cm2
= 57.96 cm–1
k = G*/R
= 57.96 cm–1/4.55 × 103 Ω
= 1.27 × 10–2 S cm–1
∧m = k × 1000/C
= [1.27 × 10–2 S cm–1] × 1000/0.05 mol/cm3
= 254.77 S cm2 mol–1
Question. (i) State the law which helps to determine the limiting molar conductivity of weak electrolyte.
(ii) Calculate limiting molar conductivity of CaSO4
(limiting molar conductivity of calcium and sulphate ions are 119.0 and 160.0 S cm2 mol–1 respectively)
Answer. Kohlrausch law of independent migration of ions:
(i) The limiting molar conductivity of an electrolyte can be represented as the sum of the individual contribution of the anions and cations of the electrolyte.
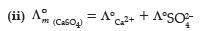
= 119.0 S cm2 mol–1 + 160.0 S cm2 mol–1
= 279.0 S cm2 mol–1
Question. A galvanic cell consists of a metallic zinc plate immersed in 0.1 M Zn(NO3)2 solution and metallic plate of lead in 0.02 M Pb(NO3)2 solution. Calculate the emf of the cell. Write the chemical equation for the electrode reactions and represent the cell.
(Given: E°Zn2+/Zn = – 0.76 V; E°Pb2+/Pb = – 0.13V)
Answer.
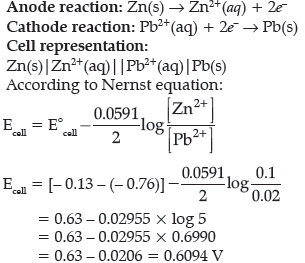
Question. Consider the following reaction:
Cu(s) + 2Ag+(aq) → 2Ag(s) + Cu2+(aq)
(i) Depict the galvanic cell in which the given reaction takes place.
(ii) Give the direction of flow of current.
(iii) Write the half-cell reactions taking place at cathode and anode.
Answer. (i) Cu(s) | Cu2+(aq) || Ag+ (aq) | Ag(s)
(ii) Current will flow from silver to copper electrode in the external circuit.
(iii) Cathode: 2Ag+(aq) + 2e– → 2Ag(s)
Anode: Cu(s) → Cu2+ (aq) + 2e–
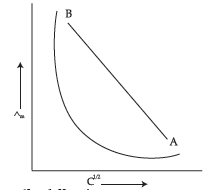
Question. The following curve is obtained when molar conductivity (∧m) is plotted against the square root of concentration, C1/2 for two electrolytes A and B:
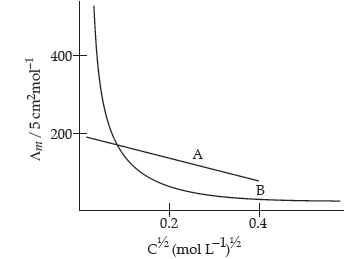
(i) How do you account for the increase in the molar conductivity of the electrolyte A on dilution ?
(ii) As seen from the graph, the value of limiting molar conductivity (L°m) for electrolyte B cannot be obtained graphically. How can this value be obtained ?
(iii) Define limiting molar conductivity.
Answer. (i) As seen from the graph, electrolyte A is a strong electrolyte which is completely ionised in solution. With dilution, the ions are far apart from each other and hence the molar conductivity increases.
(ii) To determine the value of limiting molar conductivity for electrolyte B, indirect method based upon Kohlrausch’s law of independent migration of ions is used.
(iii) When concentration approaches zero, the molar conductivity is known as limiting molar conductivity.
Question. (a) The cell in which the following reaction occurs:
2 Fe3+ (aq) + 2 I– (aq) → 2 Fe2+ (aq) + I2 (s)
has E°cell = 0.236 V at 298 K. Calculate the standard Gibb’s energy of the cell reaction.
(Given: 1 F = 96,500 C mol–1)
(b) How many electrons flow through a metallic wire if a current of 0.5 A is passed for 2 hours ?
(Given: 1 F = 96,500 C mol–1)
Answer. (a) ΔG° = – nFE°cell
n = 2
ΔG° = – 2 × 96500 C /mol × 0.236 V
= – 45548 J/mol
= – 45.548 kJ/mol
(b) Q = I t = 0.5 × 2 × 60 × 60 = 3600 C
96500 C = 6.023 × 1023 electrons
3600 C = 2.25 × 1022 electrons
Question. Calculate e.m.f. of the following cell at 298 K:
2Cr(s) + 3Fe2+ (0.1M) → 2Cr3+ (0.01M) + 3Fe(s)
E°(Cr3+ / Cr) = – 0.74 V
E° (Fe2+ / Fe) = – 0.44 V.
Answer.

Question. (i) Give Debye Huckel Onsager equation for strong electrolyte.
(ii) Given are the conductivity and molar conductivity of NaCl solutions at 298K at different concentrations:

Compare the variation of conductivity and molar conductivity of NaCl solutions on dilution. Give reason.
Answer.(i) Debye Huckel Onsager equation for strong electrolyte is:
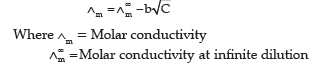
b = Constant
C = Concentration of solution
(ii) Conductivity of NaCl decreases on dilution as the number of ions per unit volume decreases.
Whereas molar conductivity of NaCl increases on dilution as on dilution the interionic interactions overcome and ions are free to move.
Question. Calculate the emf of the following cell at 25° C:
Fe | Fe2+ (0.001 M) || H+ (0.01 M) | H2(g) (1bar) | Pt(s)
E° (Fe2+ / Fe) = – 0.44 V E° (H+ / H2) = 0.00 V
Answer.

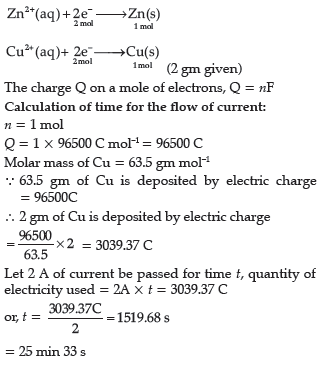
Question. When a steady current of 2A was passed through two electrolytic cells A and B containing electrolytes ZnSO4 and CuSO4 connected in series,2 g of Cu were deposited at the cathode of cell B.How long did the current flow? What mass of Zn was deposited at cathode of cell A?
[Atomic mass: Cu = 63.5 g mol–1, Zn = 65 g mol–1;1F = 96500 C mol–1]
Answer.
Question. Calculate ΔrG° and log Kc for the following reaction at 298 K.
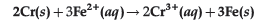
[(E°cell = 0.30 V), 1F = 96500 C mol-1]
Answer. ΔrG° = -nFE°cell, n = 6
= -6 × 96500 C/mol × 0.30 V
= -173700 J/mol = -173.7 kJ/mol
E°cell = 0.059V/n × log Kc
log Kc = 0.30 V × 6/0.059 V = 30.5
Long Answer Type Questions
Question. (a) Write the cell reaction and calculate the e.m.f. of the following cell at 298 K:
Sn (s) | Sn2+ (0.004 M) || H+ (0.020 M) | H2 (g)
(1 bar) | Pt (s)
(Given: E° Sn2+/ Sn = – 0.14V)
(b) Give reasons:
(i) On the basis of E° values, O2 gas should be liberated at anode but it is Cl2 gas which is liberated in the electrolysis of aqueous NaCl.
(ii) Conductivity of CH3COOH decreases on dilution.
Answer.

The value of E° of O2 is higher than Cl2 but O2 is evolved from H2O only when the higher voltage is applied. So, because of this Cl2 is evolved instead of O2.
(ii) Conductivity varies with the change in the concentration of the electrolyte. The number of ions per unit volume decreases on dilution. So, conductivity decreases
with decrease in concentration. Therefore,conductivity of CH3COOH decreases on dilution.
Question. The resistance of 0.01 M acetic solution when measured in a conductivity cell of cell constant 0.366 cm-1, is found to be 2220 Ω. Calculate degree of dissociation of acetic acid at this concentration.
Also find the dissociation constant of acetic acid.
Given that value of λ+ H+ and λ∞ CH3 COO– as 349.1 and 40.9 Ω-1cm2mol-1 respectively.
Answer. Conductivity(k) of 0.01 M acetic acid
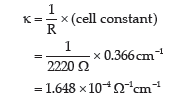

Question. Eocell for the given redox reaction is 2.71 V.
Mg + Cu2+(0.01 M) → Mg2+(0.001 M) + Cu
Calculate Ecell for the reaction. Write the direction of flow of current when an external opposite potential applied is:
(i) Less than 2.71 V
(ii) Greater than 2.71 V
Answer.
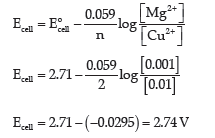
(i) When external opposite applied voltage is less than 2.71, it is less than Eocell , therefore, the electrons will flow from the anode to the cathode, and current will flow from cathode (copper electrode) to anode (magnesium electrode).
(ii) When external opposite applied potential is greater than 2.71, which is greater than Eocell ,therefore, the reaction will be reversed, and the current will flow from anode to cathode.
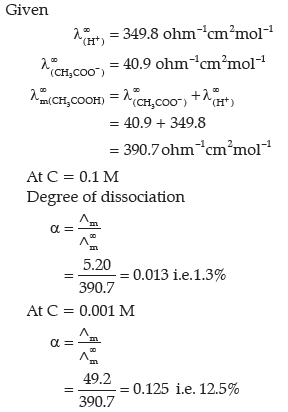
Question. The molar conductivities of acetic acid at 298 K at the concentrations of 0.1 M and 0.001 M are 5.20 and 49.2 S cm2mol-1 respectively. Calculate the degree of dissociation of acetic acid at these concentration. Given λ+( H+) λ∞( CH3 COO–) and 40.9 ohm-1cm2mol-1 respectively.
Answer.
Question. (a) Represent the cell in which the following reaction takes place:
2Al( s)+ 3Ni2+ ( 0.1M) → 2Al3+ ( 0.01M)+ 3Ni( s)
Calculate its emf if Eocell = 1.41V.
(b) How does molar conductivity vary with increase in concentration for strong electrolyte and weak electrolyte? How can you obtain limiting molar conductivity ( ∧om ) for weak electrolyte?
Answer.

(b) When the concentration of weak electrolyte becomes very low, its degree of ionization rises. This increase leads to increase in the number of ions in the solution. Thus, the molar conductivity rises sharply of a weak electrolyte at low concentration. The molar conductivity of strong electrolyte decreases a bit with an increase in concentration. This is due to increase in interionic attraction due to higher number of ions per unit volume. On dilution, ions move apart, weakening interionic attractions and thus conductance increases.
Limiting molar conductivity for weak electrolytes is obtained by using Kohlrausch law of independent migration of ions.
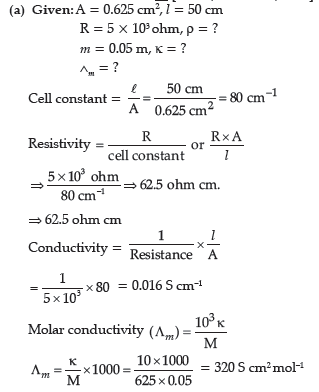
Question. (a) The electrical resistance of a column of 0.05 M KOH solution of length 50 cm and area of cross-section 0.625 cm2 is 5 × 103 ohm.
Calculate its resistivity, conductivity and molar conductivity.
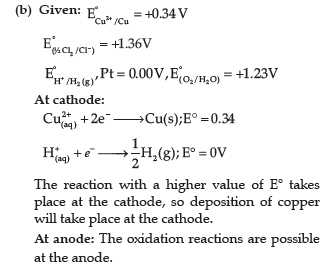
(b) Predict the products of electrolysis of an aqueous solution of CuCl2 with platinum
electrodes.
Answer.

Question. (a) Calculate E°cell for the following reaction at 298K:
2Al(s) + 3Cu2+ (0.01M) → 2Al3+ (0.01M) + 3Cu(s)
Given: E°cell = 1.98 V
(b) Using the E° values of A and B, predict which is better for coating the surface of iron [E°(Fe2+/Fe) = – 0.44V] to prevent corrosion and why ?
Given: E°(A2+/A) = – 2.37V: E°(B2+/B) = – 0.14V
Answer.
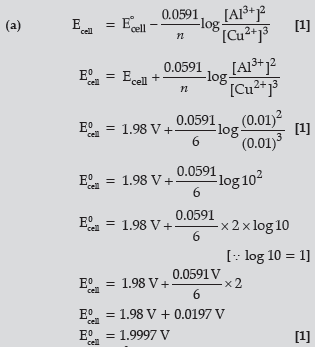
(b) A, because its E0 value is more negative
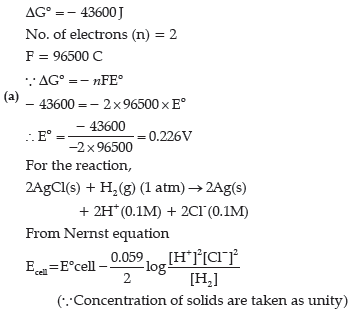
Question. (a) For the reaction
2AgCl (s) + H2 (g) (1 atm) → 2Ag(s)+2H+
(0.1 M)+2Cl-(0.1 M),
ΔG°= – 43600 J at 25°C.
Calculate the e.m.f. of the cell.
[log 10–n = –n]
(b) Define fuel cell and write its two advantages.
Answer.
Question. (a) The conductivity of 0.001 mol L–1 solution of CH3COOH is 3.905 × 10–5 S cm–1. Calculate its molar conductivity and degree of dissociation (α).
Given λ° (H+) = 349.6 S cm2 mol–1 and λ0 (CH3COO–) = 40.9 S cm2 mol–1
(b) Define electrochemical cell. What happens if external potential applied becomes greater than E°cell of electrochemical cell ?
Answer.
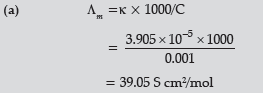
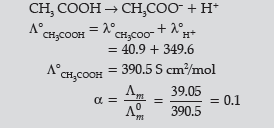
(b) Device used for the production of electricity from energy released during spontaneous chemical reaction and the use of electrical energy to bring about a chemical change.
The reaction gets reversed / It starts acting as an electrolytic cell & vice – versa.
Question. (a) A steady current of 2 amperes was passed through two electrolytic cells X and Y connected in series containing electrolytes FeSO4 and ZnSO4 until 2.8 g of Fe deposited at the cathode of cell X.
How long did the current flow? Calculate the mass
(Molar mass: Fe = 56 g mol-1, Zn = 65.3 g mol-1,1 F = 96500 C mol-1)
(b) In the plot of molar conductivity Λm vs. square root of concentration (C½), following curve obtained for two electrolytes A and B:
Answer the following:
(i) Predict the nature of electrolytes A and B:
(ii) What happens on extrapolation of Λm to concentration approaching zero for electrolytes A and B?
Answer.
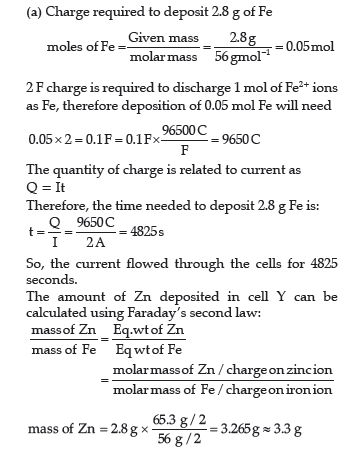
Therefore, the mass of Zn deposited in cell Y in the same time is 3.3 g.
(b) (i) Molar conductivity of strong electrolytes increases linearly as the square root of the concentration decreases; therefore, electrolyte A is a strong electrolyte. Molar conductivity of weak electrolytes increases non-linearly as square root of concentration decreases; therefore, electrolyte B is a weak electrolyte.
(ii) As concentration of strong electrolyte approaches zero, the molar conductivity of the plot intercepts the molar conductivity axis, giving the limiting value of molar conductivity Λ°m. The plot of molar conductivity of weak electrolyte tends to infinity as its concentration approaches zero; it does not intersect the molar conductivity axis.
Question. (a) Calculate e.m.f. of the following cell:
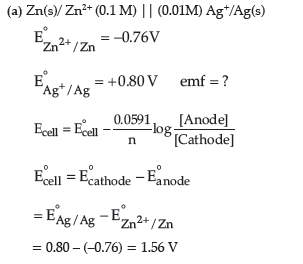
Zn(s)/Zn2+ (0.1 M) || (0.01 M) Ag+/Ag(s)
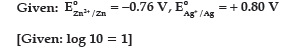
(b) X and Y are two electrolytes. On dilution molar conductivity of ‘X’ increases 2.5 times while that Y increases 25 times. Which of the two is a weak electrolyte and why?
Answer.

Question. (a) The conductivity of 0.001 mol L–1 acetic acid is 4.95 × 10–5 S cm–1. Calculate the dissociation constant if o ∧m for acetic acid is 390.5 S cm2 mol–1.
(b) Write Nernst equation for the reaction at 25°C:
2Al(S)+ 3Cu2+ (aq) → 2Al3+ (aq)+ 3Cu(s)
(c) What are secondary batteries? Give an example.
Answer.
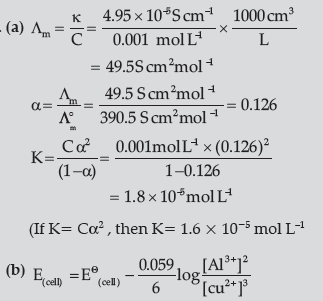
(c) Batteries which are rechargeable
Example- Lead storage, Ni-Cd batteries (Or any other one example).


