Please see below Case Study MCQ Questions Chapter 4 Carbon and Its Compound Class 10 Science. These MCQ Questions with Answers for Case study have been designed as per the latest syllabus and examination guidelines of Class 10 Science. Cased Study Based MCQ Questions for Class 10 Science are expected to come in the upcoming exams. We have provided a lot of case studies for all chapters in standard 10 science. Please solve the MCQ Questions and compare with the answers provided by our teachers.
Chapter 4 Carbon and Its Compound Class 10 Science Case Study MCQ Questions
Case-based Questions :
Read the passage and answer the given questions.
A homologous series is a series of organic compounds which belong to the same family (i.e. possess same functional group) and show similar chemical properties. The members of this
series are called homologous and differ from each other by the number of CH2 units in the main carbon chain.
Question. Which of the following is not the property of a homologous series ?
(a) They show similar chemical properties.
(b) They differ by 14 units by mass.
(c) They all contain double bond
(d) They can be represented by a general formula.
Answer
C
Question. Which of the following represent the name and formula of the 2nd member of homologous series having general formula CnH2n +2 ?
(a) Methane CH4
(b) Ethane C2H6
(c) Ethene C2H4
(d) Ethyne C2H6
Answer
B
Question. The chemical properties of which of the following compounds is similar to the butane ?
(a) Butyne
(b) Propene
(c) Propyne
(d) Pentane
Answer
D
Question. The difference between two consecutive members in a homologous series in alkanes in terms of molecular mass and number of atoms of elements is:
(a) 14 a.m.u and CH2 respectively
(b) 12 a.m.u and CH3 respectively
(c) 14 a.m.u and CH respectively
(d) 12 a.m.u and CH3 respectively
Answer
A
Read the given passage and answer the questions given below :
Carbon has the unique property to form bonds with other atoms of carbon.
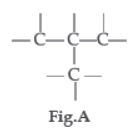
Question. Carbon is :
(a) Divalent
(b) Monovalent
(c) Tetravalent
(d) Trivalent
Answer
C
Question. Write the name and structure of a saturated compound in which 6 carbon atoms are arranged in a ring.
(a) Hexane
(b) Cyclohexane
(c) Pentane
(d) Cyclopentane
Answer
B
Question. Name the characteristic property of carbon as depicted in the fig.
(a) Catenation
(b) Polymerisation
(c) Isomerisation
(d) None of the above.
Answer
A
Question. Carbon forms large number of compounds due to :
(a) Catenation only
(b) Tetravalency only
(c) Both catenation and tetravalency
(d) None of the above
Answer
C
Read the passage and answer the questions given below :
Homologous series is a series of compounds with similar chemical properties and same functional group differing from the successive member by —CH2 unit. Carbon chains of varying length have been observed in organic compounds having the same general formula. Such organic compounds that vary from one another by a repeating unit and have the same general formula form a series of compounds. Alkanes with general formula CnH2n+2, alkenes with general formula CnH2n and alkynes with general formula CnH2n – 2 form the most basic homologous series in organic chemistry.
All the members belonging to this series have the same functional groups. They have similar physical properties and follow a fixed gradation with increasing mass. This series has enabled scientists to study different organic compounds systematically. They can predict the properties of organic compounds belonging to a particular homologous series based on the data available from the other members of the same series. The study of organic compounds has been simplified.
Question. What is the molecular formula of the 5th member of the homologous series of carbon compounds is represented by the general formula CnH2n+1OH ?
(a) C5H10
(b) C5H11OH
(c) C5H12OH
(d) C5H11CHO
Answer
B
Question. The general formula for alkene is :
(a) CnH2n
(b) CnH2n+2
(c) CnH2n – 2
(d) CnH2n +1
Answer
A
Question. Which of these statements is correct about the members of a homologous series ?
(a) They have same empirical formula.
(b) They have same general formula.
(c) They have same molecular formula.
(d) They have same physical properties.
Answer
B
Question. Two compounds CH3OH and C2H5OH are provided. The difference in its formulae and molecular masses are ___I___ and ____II____.
(a) I- CH3, II- 16 units
(b) I- CH2, II- 14 units
(c) I- CH4, II- 18 units
(d) I – CH3, II- 16 units
Answer
B
Reactions in which an atom or a group of atoms is replaced by some other atom or another group of atoms without causing any change in the structure of the remaining part of the molecule, are called substitution reactions. All organic compounds containing double or triple bonds give addition reactions, i.e., alkenes, alkynes and aromatic hydrocarbons give addition reactions. Reactions in which the compounds react with oxygen and form carbon dioxide and water is known as combustion reaction. This process occurs with release of great amount of heat.
Question. The reaction CH2 = CH2 + H2 → CH3 – CH3 is :
(a) substitution reaction
(b) addition reaction
(c) rearrangement reaction
(d) elimination reaction
Answer
B
Question. The reaction C2H6 + O2 → 2CO2 + 3H2O is :
(a) substitution reaction
(b) rearrangement reaction
(c) addition reaction
(d) combustion reaction
Answer
D
Question. The reaction CH4 + Cl2 → CH3Cl + HCl is :
(a) substitution reaction
(b) addition reaction
(c) rearrangement reaction
(d) elimination reaction
Answer
A
The given diagram represent an experiment in which a test tube contains 1 mL of ethanol (absolute alcohol) and 1 mL glacial acetic acid along with a few drops of concentrated H2SO4. Observe the diagram and answer the following questions.

Question. Write the chemical equation.
Answer
CH3 COOH + CH3 –CH2 OH Acid →
Ethanoic acid Ethanol CH3 – C – O – CH2 –CH
ll
O
Question. Why reverse of this reaction is known as saponification reaction?
Answer
Reverse reaction is known as saponification reaction because it is used in the prepration of soap.
Question. Name the type of reaction taking place in this experiment.
Answer
Esterification reaction
Question. Give two uses of the resulting product.
Answer
Esters are used in making perfumes and as a flavouring agent.
Food, clothes, medicines, books, or many of the things are all based on this versatile element carbon. In addition, all living structures are carbon based. The earth’s crust has only 0.02% carbon in the form of minerals. The element carbon occurs in different forms in nature with widely varying physical properties. Both diamond and graphite are formed by carbon atoms, the difference lies in the manner in which the carbon atoms are bonded to one another. Carbon has the unique ability to form bonds with other atoms of carbon, giving rise to large molecules. This property is called catenation.
Question. Which of the following are isomers?
(a) Butane and isobutene
(b) Ethane and ethene
(c) Propane and propyne
(d) Butane and isobutane
Answer
D
Question. Which one of the following is not an allotrope of carbon?
(a) Soot
(b) Graphite
(c) Diamond
(d) Carborundum
Answer
D
Question. Pentane has the molecular formula C5H12. It has
(a) 5 covalent bonds
(b) 12 covalent bonds
(c) 16 covalent bonds
(d) 17 covalent bonds
Answer
C
QuestionFrom the given alternatives, whose chemical and physical properties are not same?
(a) Graphite and Diamond
(b) Phosphorous and Sulphur
(c) Carbon and Hydrogen
(d) Methyl alcohol and Acetic acid
Answer
D
Question. Which of the following statements is not correct?
(a) Graphite is much less dense than diamond
(b) Graphite is black and soft
(c) Graphite has low melting point
(d) Graphite feels smooth and slippery
Answer
C
A carbon atom attached to one, two, three and four other carbon atoms is called primary, secondary, tertiary and quaternary carbon respectively. Now consider following compound and answer the following questions.
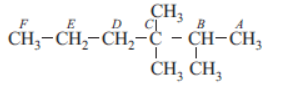
Question. In above compound which carbon atom is quaternary?
(a) B
(b) D
(c) F
(d) C
Answer
D
Question. In above compound how many carbon atom are primary?
(a) 7
(b) 5
(c) 6
(d) 4
Answer
B
Question. In above compound how many carbon atoms are secondary?
(a) 2
(b) 1
(c) 3
(d) 0
Answer
A



