Please refer to Assignments Class 12 Chemistry Biomolecules Chapter 14 with solved questions and answers. We have provided Class 12 Chemistry Assignments for all chapters on our website. These problems and solutions for Chapter 14 Biomolecules Class 12 Chemistry have been prepared as per the latest syllabus and books issued for the current academic year. Learn these solved important questions to get more marks in your class tests and examinations.
Biomolecules Assignments Class 12 Chemistry
Question. Glucose and fructose are:
(A) isomers of each other
(B) Homologous of each other
(C) anomers of each other
(D) enantiomers of each other
Answer
A
Question. Lysine
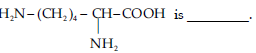
(A) a-Amino acid.
(B) Basic amino acid.
(C) Amino acid synthesised in body.
(D) b-Amino acid.
Answer
A,B,C
Question. Amino acids are:
(A) Acidic
(B) Basic
(C) Amphoteric
(D) Neutral
Answer
C
Question. Proteins are found to have two different types of secondary structures, viz. a-helix and β-pleated sheet structure. a-helix structure of protein is stabilised by:
(A) Peptide bonds
(B) van der Waals forces
(C) Hydrogen bonds
(D) Dipole-dipole interactions
Answer
C
Question. Three cyclic structures of monosaccharides are given below which of these are anomers ?
(A) (i) and (ii)
(B) (ii) and (iii)
(C) (i) and (iii)
(D) (iii) is anomer of (i) and (ii)
Answer
A
Question. Which one is the complementary base of cytosine in one strand to that in other strand of DNA?
(A) Adenine
(B) Guanine
(C) Thymine
(D) Uracil
Answer
B
Question. Curdling of milk is an example of:
(A) breaking of peptide linkage
(B) hydrolysis of lactose
(C) breaking of protein into amino acids
(D) denaturation of protein
Answer
D
Question.1. Which of the following pairs represents anomers?
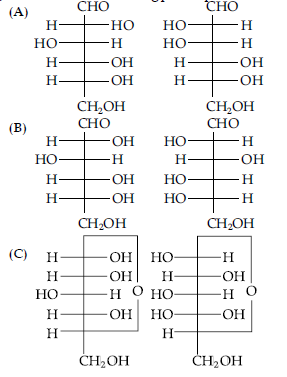

Answer
C
Question. Dinucleotide is obtained by joining two nucleotides together by phosphodiester linkage.Between which carbon atoms of pentose sugars of nucleotides are these linkages present ?
(A) 5’ and 3’
(B) 1’ and 5’
(C) 5’ and 5’
(D) 3’ and 3’
Answer
A
Question. Which of the following is an example of aldohexose?
(A) Ribose
(B) Fructose
(C) Sucrose
(D) Glucose
Answer
D
ASSERTION AND REASON BASED MCQs
Directions: In the following questions, A statement of Assertion (A) is followed by a statement of Reason (R). Mark the correct choice as.
(A) Both A and R are true and R is the correct explanation of A
(B) Both A and R are true but R is NOT the correct explanation of A
(C) A is true but R is false
(D) A is false and R is True
Question. Assertion (A): D(+)–Glucose is dextrorotatory in nature.
Reason (R): ‘D’ represents its dextrorotatory nature.
Answer
C
Question. Assertion (A): Deoxyribose, C5H10O4 is not a carbohydrate.
Reason (R): Carbohydrates are optically active polyhydroxy aldehyde or polyhydroxy ketone or substances which give aldehyde or ketone on hydrolysis.
Answer
D
Question. Assertion (A): Glucose reacts with hydroxylamine to form an oxime and also adds a molecule of hydrogen cyanide to give cyanohydrin.
Reason (R): The carbonyl group is present in the openchain structure of glucose.
Answer
A
Question. Assertion (A): The two strands of DNA are complementary to each other.
Reason (R): The hydrogen bonds are formed between specific pairs of bases.
Answer
A
Question. Assertion (A): All naturally occurring α amino acids except glycine are optically active.
Reason (R): Most naturally occurring α amino acids have L-configuration.
Answer
B
Question. Assertion (A): Glycine must be taken through diet.
Reason (R): It is non-essential amino acid.
Answer
D
CASE-BASED MCQs
I. Read the passage given below and answer the following questions:
EVIDENCE FOR THE FIBROUS NATURE OF DNA
The basic chemical formula of DNA is now well established. As shown in Figure 1 it consists of a very long chain, the backbone of which is made up of alternate sugar and phosphate groups, joined together in regular 3’ 5’ phosphate di-ester linkages. To each sugar is attached a nitrogenous base, only four different kinds of which are commonly found in DNA. Two of these—adenine and guanine— are purines, and the other two thymine and cytosine-are pyrimidines. A fifth base, 5-methyl cytosine, occurs in smaller amounts in certain organisms, and a sixth, 5-hydroxy-methyl-cytosine, is found instead of cytosine in the T even phages. It should be noted that the chain is unbranched, a consequence of the regular internucleotide linkage. On the other hand the sequence of the different nucleotides is, as far as can be ascertained, completely irregular. Thus, DNA has some features which are regular, and some which are irregular. A similar conception of the DNA molecule as a long thin fiber is obtained from physicochemical analysis involving sedimentation, diffusion, light scattering, and viscosity measurements. These techniques indicate that DNA is a very asymmetrical structure approximately 20 A wide and many thousands of angstroms long. Estimates of its molecular weight currently center between 5 X106 and X107 (approximately 3 X104 nucleotides). Surprisingly each of these measurements tend to suggest that the DNA is relatively rigid, a puzzling finding in view of the large number of single bonds (5 per nucleotide) in the phosphate-sugar back bone. Recently these indirect inferences have been confirmed by electron microscopy.
Question. DNA has a ___________ backbone
(A) phosphate-purine
(B) pyrimidines-sugar
(C) phosphate-sugar
(D) purine-pyrimidine
Answer
C
Question. DNA molecule has ___________ internucleotide linkage and __________ sequence of the different nucleotides
(A) regular, regular
(B) regular, irregular
(C) irregular, regular
(D) irregular, irregular
Answer
B
Question. Purines present in DNA are:
(A) adenine and thymine
(B) guanine and thymine
(C) cytosine and thymine
(D) adenine and guanine
Answer
D
Question. Out of the four different kinds of nitrogenous bases which are commonly found in DNA, ___________ has been replaced in some organisms.
(A) adenine
(B) guanine
(C) cytosine
(D) thymine
Answer
C
II. Read the passage given below and answer the following questions:
The two monosaccharides are joined together by an oxide linkage formed by the loss of a water molecule. Such a linkage between two monosaccharide units through oxygen atom is called glycosidic linkage. In disaccharides, if the reducing groups of monosaccharides i.e., aldehydic or ketonic groups are bonded, these are non-reducing sugars, e.g., sucrose. On the other hand, sugars in which these functional groups are free, are called reducing sugars, for example, maltose and lactose. A non reducing disaccharide ‘A’ on hydrolysis with dilute acid gives an equimolar mixture of D–(+)– glucose and D-(-)-Fructose.
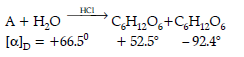
Question. What is the mixture of D-(+) glucose and D-(+) fructose known as ?
(A) Anomers
(B) Racemic mixture
(C) Invert sugar
(D) Optical mixture
Answer
C
Question. Glucose on reaction with acetic acid gives glucose pentaacetate. What does it suggest about the structure of glucose ?
(A) C-1 is anomeric carbon
(B) C-5 is anomeric carbon
(C) 3’-OH groups are present
(D) 5’-OH groups are present
Answer
D
Question. In the above reaction, reactant ‘A’ is:
(A) Glucose
(B) Sucrose
(C) Maltose
(D) Fructose
Answer
B
Question. Name the linkage that holds the two units in the disaccharide ?
(A) Nucleoside linkage
(B) Glycosidic linkage
(C) Peptide linkage
(D) None of the above
Answer
B
III. Read the passage given below and answer the following questions:
The sequence of bases in m-RNA are read in a serial order in groups of three at a time. Each triplet of nucleotides (having a specific sequence of bases) is known as codon. Each codon specifies one amino acid. Many amino acids have more than one codons. The amino acids are brought to the mRNA by another type of RNA and called tRNA. Each amino acid has atleast one corresponding tRNA. At one end of the tRNA molecule is a trinucleotide base sequence that is complementary to some trinucleotide base sequence on mRNA.
Question. In mRNA, the complementary bases of AAT is:
(A) CCG
(B) UUA
(C) AUU
(D) UUU
Answer
B
Question. Each triplet of nucleotides is called:
(A) Anticodon
(B) Codon
(C) mRNA
(D) tRNA
Answer
B
Question. Each codon specifies:
(A) 1 amino acid
(B) 2 amino acids
(C) 3 amino acids
(D) None of these
Answer
A
Question. Which of the following nitrogen bases is not present in RNA?
(A) Thymine
(B) Adenine
(C) Guanine
(D) Cytosine
Answer
A
IV. Read the passage given below and answer the following questions:
Adenosine triphosphate (ATP) is the energycarrying molecule found in the cells of all living things. ATP captures chemical energy obtained from the breakdown of food molecules and releases it to fuel other cellular processes. ATP is a nucleotide that consists of three main structures: the nitrogenous base, adenine; the sugar, ribose; and a chain of three phosphate groups bound to ribose. The phosphate tail of ATP is the actual power source which the cell taps. Available energy is contained in the bonds between the phosphates and is released when they are broken, which occurs through the addition of a water molecule (a process called hydrolysis). Usually only the outer phosphate is removed from ATP to yield energy; when this occurs ATP is converted to adenosine diphosphate (ADP), the form of the nucleotide having only two phosphates.
The importance of ATP (adenosine triphosphate) as the main source of chemical energy in living matter and its involvement in cellular processes has long been recognized. The primary mechanism whereby higher organisms, including humans, generate ATP is through mitochondrial oxidative phosphorylation. For the majority of organs, the main metabolic fuel is glucose, which in the presence of oxygen undergoes complete combustion to CO2 and H2O:
C6H12O6 + 6O2 → 6O2 + 6H2O + energy The free energy (ΔG) liberated in this exergonic (ΔG is negative) reaction is partially trapped as ATP in two consecutive processes: glycolysis (cytosol) and oxidative phosphorylation (mitochondria). The first produces 2 mol of ATP per mol of glucose, and the second 36 mol of ATP per mol of glucose. Thus, oxidative phosphorylation yields 17-18 times as much useful energy in the form of ATP as can be obtained from the same amount of glucose by glycolysis alone. The efficiency of glucose metabolism is the ratio of amount of energy produced when 1 mol of glucose oxidised in cell to the enthalpy of combustion of glucose. The energy lost in the process is in the form of heat. This heat is responsible for keeping us warm.
The following questions are multiple choice questions. Choose the most appropriate answer:
Question. What is the efficiency of glucose metabolism if 1 mole of glucose gives 38ATP energy? (Given: The enthalpy of combustion of glucose is 686 kcal, 1ATP = 7.3kcal)
(A) 100%
(B) 38%
(C) 62%
(D) 80%
Answer
B
Question. Which of the following statements is correct:
(A) ATP is a nucleotide which has three phosphate groups while ADP is a nucleoside which three phosphate groups.
(B) ADP contains a nitrogenous bases adenine,ribose sugar and two phosphate groups bound to ribose.
(C) ADP is the main source of chemical energy in living matter.
(D) ATP and ADP are nucleosides which differ in number of phosphate groups.
Answer
B
Question. Which of the following statement is true?
(A) ATP is a nucleoside made up of nitrogenous base adenine and ribose sugar.
(B) ATP consists the nitrogenous base, adenine and the sugar, deoxyribose.
(C) ATP is a nucleotide which contains a chain of three phosphate groups bound to ribose sugar.
(D) The nitrogenous base of ATP is the actual power source.
Answer
C
Question. Cellular oxidation of glucose is a:
(A) spontaneous and endothermic process
(B) non spontaneous and exothermic process
(C) non spontaneous and endothermic process
(D) spontaneous and exothermic process
Answer
D
Question. Nearly 95% of the energy released during cellular respiration is due to:
(A) glycolysis occurring in cytosol
(B) oxidative phosphorylation occurring in cytosol
(C) glycolysis in occurring mitochondria
(D) oxidative phosphorylation occurring in mitochondria
Answer
D
Very Short Answer Type Questions
Question. How would you explain the amphoteric behavior of amino acids?
Answer.Amino acids are amphoteric due to the presence of both acidic and basic functional groups.
Question. Name the linkage present in proteins.
Answer. Peptide linkage
Question. Give the significance of prefix ‘D’ in the name D- (+)-glucose.
Answer.‘D’ Signifies that –OH group on C-5 is on the right-hand side
Question. How many asymmetric carbon atoms are present in D (+) glucose?
Answer. 4
Question. Write the Zwitter ion form of amino acetic acid. (H2NCH2COOH).
Answer.NH3+CH2COO–
Question. Mention the number of hydrogen bonds between adenine and thymine.
Answer.Two
Question. What type of linkage holds together the monomers of DNA and RNA?
Answer.Phosphodiester linkage.
Question. Give the significance of (+)-sign in the name D- (+)-glucose.
Answer. (+) sign indicates dextrorotatory nature of glucose.
Question.Which nucleic acid is responsible for carrying out protein synthesis in the cell?
Answer.RNA
Question.The two strands in DNA are not identical but complementary. Explain.
Answer. complementary bases are prepared.
Question. Name the deficiency diseases resulting from lack of Vitamins A and E in the diet.
Answer. Deficiency of Vitamin A causes Xerophthalmia and deficiency of Vitamin E causes Sterility.
Question. What are the products of hydrolysis of sucrose?
Answer. Invert sugar: An equimolar mixture of glucose and fructose is obtained by hydrolysis of sucrose in presence of an acid such as dil. HCl or the enzyme invertase or sucrase and is called invert sugar.
Question. Which of the two components of starch is water soluble?
Answer. Amylose is water soluble component of starch.
Question. Write the name of linkage joining two amino acids.
Answer. Peptide linkage joins two amino acids.
Question. What is meant by ‘reducing sugars’?
Answer. Reducing sugar contains aldehydic or ketonic group in the hemiacetal and hemiketal forms and can reduce Tollen’s reagent or Fehling’s solution.
Question. What are monosaccharides?
Answer. These are the simplest carbohydrates which cannot be hydrolysed to smaller molecules. Their general
formula is (CH2O)n where n = 3 – 7
Example : glucose, fructose etc.
Question. Name a water soluble vitamin which is a powerful antioxidant. Give its one natural source.
Answer. Water soluble vitamin : Vitamin C Natural source : Amla
Question. What are the products of hydrolysis of sucrose?
Answer. Glucose and fructose.
Question. Name one oil soluble vitamin which is a powerful antioxidant and give its one natural source.
Answer. Oil soluble Vitamine : Vitamin D Natural source : Fish liver oil, butter, milk, eggs etc.
Question. What are the products of hydrolysis of maltose?
Answer. Maltose hydrolysis → 2 molecules of glucose
Question. Name the products of hydrolysis of lactose.
Answer. Lactose on hydrolysis with dilute acids gives an equimolar mixture of D-glucose and D-galactose.
Short Answer Type Questions
Question.(i) What products would be formed when a nucleotide from DNA containing thymine is hydrolyzed?
Ans. Complete hydrolysis of DNA yields a pentose sugar, phosphoric acid and thymine.
(ii)How will you distinguish 1° and 2° hydroxyl groups present in glucose?
Ans- On oxidation with nitric acid, glucose as well as gluconic acid both yield a dicarboxylic acid,
Question. Explain the term mutarotation?
(Ans) Mutarotation is the change in the specific rotation of an optically active compound with time, to an equilibrium mixture.
Question. (a) How are hormones and vitamins different in respect of their source and functions?
(b) Give one example each of
(i) Globular protein (ii) Fibrous protein
Answer. (a) Hormones are synthesized in our body and help in regulation of our body systems while vitamins are synthesized artificially in the laboratory or obtained from the food which helps in controlling many diseases.
(b) (i) Globular protein : All enzymes and hormones like insulin.
(ii) Fibrous protein : Keratin in skin, nails etc.
Question. (a) What are essential and non-essential amino acids? Give two examples of each type.
(b) What are the hydrolysis products of sucrose?
Answer. (a) Non-essential amino acids : The amino acids which can be synthesised in the body, are known as non-essential amino acids.
Example : Glycine, Alanine etc.
Essential amino acids : The amino acids which cannot be synthesised in the body and must be obtained through diet are known as essential amino acids.
Example : Valine, Leucine etc.
(b) Sucrose on hydrolysis gives equimolar mixture of D(+)-glucose and D(–) fructose
C12H22O11 + H2O → C6H12O6 + C6H12O6
glucose fructose
Question. How are vitamins classified? Name the vitamin responsible for the coagulation of blood.
Answer. Vitamins are classified into two types :
(i) Water insoluble vitamins : These are fat soluble substances E.g. Vitamin A, D, E and K.
(ii) Water soluble vitamins : These include Vitamin B-Complex and Vitamin C (except B12).Vitamin K or phylloquinone is responsible for the coagulation of blood.
Question. (i) Write the name of two monosaccharides obtained on hydrolysis of lactose sugar.
(ii) Why Vitamin C cannot be stored in our body?
(iii) What is the difference between a nucleoside and nucleotide?
Answer. (i) On hydrolysis, lactose gives β-D-galactose and β-D-glucose.
(ii) Vitamin C is mainly ascorbic acid which is water soluble and is readily excreted through urine and thus cannot be stored in the body.
(iii) Nucleoside. A nucleoside contains only two basic components of nucleic acids, i.e., a pentose sugar and a nitrogenous base. It is formed by the attachment of a base to 1′ position of sugar.
Question. (i) Deficiency of which vitamin causes nightblindness?
(ii) Name the base that is found in nucleotide of RNA only.
(iii) Glucose on reaction with HI gives nhexane. What does it suggest about the structure of glucose?
Answer. (i) Vitamin A causes night blindness.
(ii) Uracil is found in nucleotide of RNA only.
(iii) It suggests the open structure of glucose.
Question. What are the different types of RNA found in cells of organisms ? State the functions of each type.
Answer. Different types of RNA found in the cell are :
1. Messenger RNA (mRNA) : carries the message of DNA for specific protein synthesis.
2. Ribosomal RNA (rRNA) : provides the site for protein synthesis.
3. Transfer RNA (t-RNA) : transfers amino acids to the site of protein synthesis.
Question. (i) Deficiency of which vitamin causes scurvy?
(ii) What type of linkage is responsible for the formation of proteins?
(iii) Write the product formed when glucose is treated with HI.
Answer. (i) Vitamin C causes scurvy.
(ii) Peptide linkages are responsible for the formation of proteins.
(iii) Glucose is treated with HI :
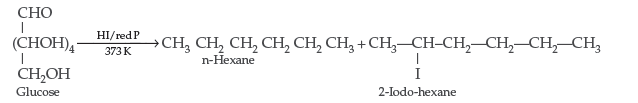
Question. Define the following as related to proteins :
(i) Peptide linkage (ii) Primary structure
(iii) Denaturation
Answer. (i) Peptide linkage : Two amino acids of same type or different types combine together by the elimination of H2O molecule to form – CONH- linkage.
(ii) Primary structure : It refers to the sequence in which amino acids are joined.
(iii) Denaturation : When a native protein is subjected to change in temperature or pH, hydrogen bonds get disturbed and globules get uncoiled and proteins lose their biological activity.
Question. Amino acids may be acidic, alkaline or neutral.How does this happen? What are essential and non-essential amino acids? Name one of each type.
Answer. Amino acids can be broadly classified into three classes i.e. acidic, alkaline and neutral amino acids depending on the number of —NH2 group and — COOH group.
Acidic amino acids : Those α-amino acids such as aspartic acid, asparagine and glutamic acid which contain two –COOH groups and one –NH2 group are called acidic amino acids.
Alkaline or Basic amino acids : Those α-amino acids such as lysine, arginine and histidine which contain two –NH2 groups and one –COOH group, are called basic amino acids.
Neutral amino acids : Those α-amino acids such as glycine, alanine, valine etc. which contain one –NH2 and one – COOH group, are called neutral amino acids.
Essential and non-essential amino acids :


