Please refer to Assignments Class 12 Physics Dual Nature of Radiation and Matter Chapter 11 with solved questions and answers. We have provided Class 12 Physics Assignments for all chapters on our website. These problems and solutions for Chapter 11 Dual Nature of Radiation and Matter Class 12 Physics have been prepared as per the latest syllabus and books issued for the current academic year. Learn these solved important questions to get more marks in your class tests and examinations.
Dual Nature of Radiation and Matter Assignments Class 12 Physics
Question: Monochromatic light of frequency 6 × 1014 Hz is produced by a laser. The power emitted is 2 × 10–3 W. The number of photons emitted per second is (Given h = 6.63 × 10–34 J s)
(a) 2 × 1015
(b) 3 × 1015
(c) 4 × 1015
(d) 5 × 1015
Answer:
D
Question: The photoelectric cut off voltage in a certain experiment is 1.5 V. The maximum kinetic energy of photoelectrons emitted is
(a) 2.4 eV
(b) 1.5 eV
(c) 3.1 eV
(d) 4.5 eV
Answer:
B
Question: The matter-wave picture of electromagnetic wave/radiation elegantly incorporated the
(a) Heinsenberg’s uncertainty principle
(b) correspondence principle
(c) cosmic theory
(d) Hertz’s observations
Answer:
A
Question: In an accelerator experiment on high energy collisions of electrons with positrons, a certain event is interpreted as annihilation of an electron-positron pair of total energy 10.2 × 109 eV into two g-rays of equal energy. The wavelength associated with each g-ray is
(a) 3.21 × 10–18 m
(b) 1.23 × 10–16 m
(c) 2.44 × 10–16 m
(d) 4.21 × 10–14 m
Answer:
C
Question: A metallic surface is irradiated by a monochromatic light of frequency u1 and stopping potential is found to be V1. If the light of frequency u2 irradiates the surface, the stopping potential will be
(a) V1+h/e (v1 + v2 )
(b) V1+/h/e (v2-v1 )
(c) V1+/e/h(v2-v1)
(d) V1-h/e (v1+v2 )
Answer:
B
Question: The wavelength of a photon needed to remove a proton from a nucleus which is bound to the nucleus with 1 MeV energy is nearly
(a) 1.2 nm
(b) 1.2 × 10–3 nm
(c) 1.2 × 10–6 nm
(d) 1.2 × 10–5 nm
Answer:
B
Question: The rest mass of photon is
(a)hν/c
(b)hν/c2
(c)hνλ
(d) zero
Answer:
D
Question: A particle of mass 4m at rest decays into two particles of masses m and 3m having nonzero velocities. The ratio of the de Broglie wavelengths of the particles 1 and 2 is
(a)1/2
(b)1/4
(c) 2
(d) 1
Answer:
D
Question: A blue lamp mainly emits light of wavelength 4500 Å. The lamp is rated at 150 W and 8% of the energy is emitted as visible light. The number of photons emitted by the lamp per second is
(a) 3 × 1019
(b) 3 × 1024
(c) 3 × 1020
(d) 3 × 1018
Answer:
A
Question: The de Broglie wavelength of an electron with kinetic energy 120 eV is (Given h = 6.63 × 10–34 J s, me = 9 × 10–31 kg, 1 eV = 1.6 × 10–19 J)
(a) 2.13 Å
(b) 1.13 Å
(c) 4.15 Å
(d) 3.14 Å
Answer:
B
Question: The photoelectric threshold frequency of a metal is u. When light of frequency 4u is incident on the metal, the maximum kinetic energy of the emitted photoelectron is
(a) 4hν
(b) 3hν
(c) 5hν
(d)5hν/2
Answer:
B
Question: If h is Planck’s constant, the momentum of a photon of wavelength 0.01 Å is
(a) 10–2h
(b) h
(c) 102h
(d) 1012h
Answer:
D
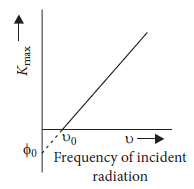
Question: According to Einstein’s photoelectric equation, the graph between the kinetic energy of photoelectrons ejected and the frequency of incident radiation is
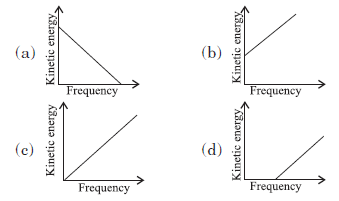
Answer:
D
Question: The de Broglie wavelength is given by
(a) p = 2πh/λ
(b) p = h/2λ
(c) p =2π/hλ
(d) p =2π/λ
Answer:
A
Question: An electron is moving with an initial velocity →ν= ν0î and is in a magnetic field →B = B0ĵ, . Then its de Broglie wavelength
(a) remains constant
(b) increases with time
(c) decreases with time
(d) increases and decreases periodically
Answer:
A
Question: A photon of energy E ejects a photoelectron from a metal surface whose work function is Φ0. If this electron enters into a uniform magnetic field B in a direction perpendicular to the field and describes a circular path of radius r, then the radius r is (in the usual notation)
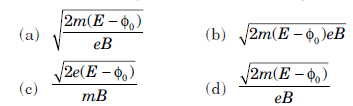
Answer:
D
Question: A and B are two metals with threshold frequencies 1.8 × 1014 Hz and 2.2 × 1014 Hz.
Two identical photons of energy 0.825 eV each are incident on them. Then photoelectrons are emitted in (Take h = 6.6 × 10–34 J s)
(a) B alone
(b) A alone
(c) neither A nor B
(d) both A and B
Answer:
B
Question: The photoelectric threshold wavelength for silver is l0. The energy of the electron ejected from the surface of silver by an incident wavelength λ(λ < λ0) will be image
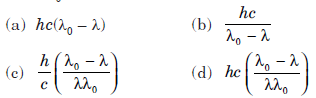
Answer:
A
Question: In photoelectric effect, the photoelectric current is independent of
(a) intensity of incident light
(b) potential difference applied between the two electrodes
(c) the nature of emitter material
(d) frequency of incident light
Answer:
D
Question: If the kinetic energy of the particle is increased by 16 times, the percentage change in the de Broglie wavelength of the particle is
(a) 25%
(b) 75%
(c) 60%
(d) 50%
Answer:
B
Question: The work function for Al, K and Pt is 4.28 eV, 2.30 eV and 5.65 eV respectively. Their respective threshold frequencies would be
(a) Pt > Al > K
(b) Al > Pt > K
(c) K > Al > Pt
(d) Al > K > Pt
Answer:
A
Question: Electrons with de Broglie wavelength λ fall on the target in an X ray tube. The cut off wavelength (λ0)of the emitted X rays is
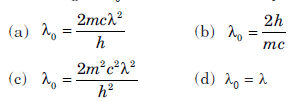
Answer:
A
Very Short Answer Type Questions
Question: There are materials which absorb photons of shorter wavelength and emit photons of longer wavelength. Can there be stable substances which absorb photons of larger wavelength and emit light of shorter wavelength.
Answer : In case of stable material, this is not possible because, to absorb a photon of larger wavelength and emit a photon of shorter wavelength, energy has to be supplied by the material.
Question: Define the term “Intensity” in photon picture of electromagnetic radiation.
Answer : The amount of light energy or photon energy, incident per unit area per unit time is called intensity of electromagnetic radiation.
Question: Name the phenomenon which shows the quantum nature of electromagnetic radiation.
Answer : Photoelectric effect shows the quantum nature of electromagnetic radiation.
Question: One milliwatt of light of wavelength
λ= 4560 Å is incident on a cesium metal surface.
Calculate the electron current liberated. Assume a quantum efficiency of h = 0.5%. [Work function for cesium = 1.89 eV]. Take hc = 12400 eV Å.
Answer: Since hc/λ =12400/4560=2.7 eV 1.89 eV so photocurrent will flow.
Number of photons incident per second
= P(Power)/Eλ(Energy of photon)P/hc=Pλ /hc
Number of photoelectron emitted per second = 0.5 [Pλ/hc]
Photocurrent =0. 5/100[Pλ/hc]e=1. 84 10-6A .
Question: A proton and an electron have same kinetic energy. Which one has greater de-Broglie wavelength and why?
Answer : We know the relation, λ = h/p
kinetic energy, K=p2/2m
Then, λ = h/√2mK
Kp = Ke ⇒ λ ∝1/√m
∴ mp >> me ∴ λp << λe
Hence for same kinetic energy wavelength associated with electron will be greater.
Short Answer Type Questions
Question: Two monochromatic beams A and B of equal intensity I, hit a screen. The number of photons hitting the screen by beam A is twice that by beam B. Then what inference can you make about their freque cies?
Answer: Let n1, n2 be the number of photons hitting the screen per second by beam A and B respectively
Intensity of beam of photon, I = nhu
∴n1u1 = n2u2
n1/n2=u2/u1
As n1/n2 = 2 ∴ u2/u1=2, u2 = 2u1
i.e, frequency of beam B is twice of that of beam A.
Question: In an experiment on photoelectric emission,following observations were made 1. Wavelength of the incident light = 2 × 10–7 m
Stopping potential = 3 V Find
(i) kinetic energy of photoelectrons with maximum speed
(ii) work function and
(iii) threshold frequency (h = 6.62 × 10–34 J s).
Answer:
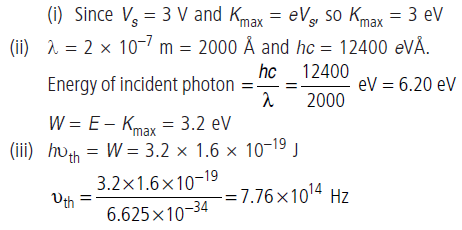
Question: (a) Ultraviolet light of wavelength 2271 Å from a 100 W mercury source is incident on a photocell made of molybdenum metal. If the stopping potential is 1.3 V, estimate the work function of the metal.
Dual Nature of Radiation and Matter 71
(b) How would the photocell respond to high intensity (105 W/m2) red light of wavelength 6328 Å produced by a He – Ne laser?
Answer:
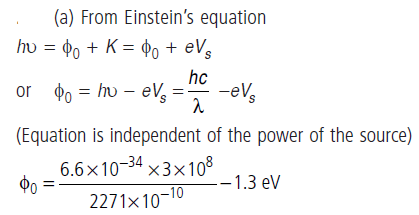

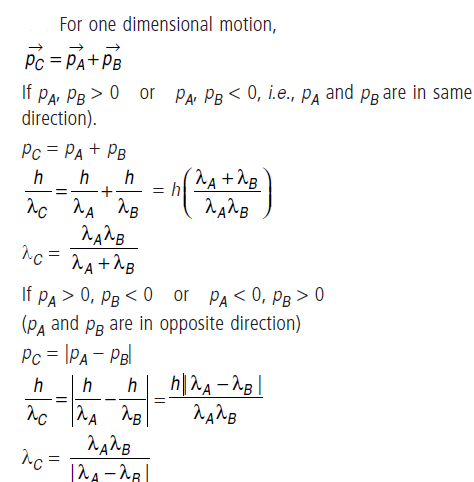
Question: Two particles A and B of de–Broglie wavelengths λ1 and λ2 combine to form a particle C. The process conserves momentum.
Find the de–Broglie wavelength of the particle C. (The motion is one dimensional).
Answer:
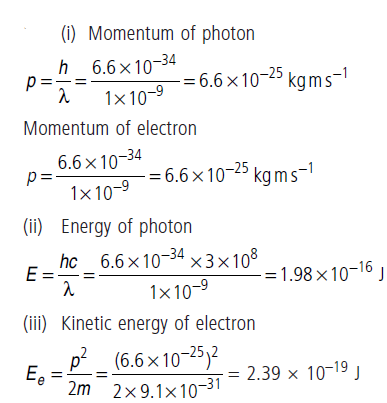
Question: An electron and a photon each have a wavelength 1.00 nm. Find
(i) their momenta,
(ii) the energy of the photon and
(iii) the kinetic energy of electron.
Answer:
Question: State three important properties of photons which describe the particle picture of electromagnetic radiation.
Answer: Photons : According to Planck’s quantum theory of radiation, an electromagnetic wave travels in the form of discrete packets of energy called quanta.
The main features of photons are as follows:
(i) In the interaction of photons with free electrons, the entire energy of photon is absorbed.
(ii) Energy of photon is directly proportional to frequency.
Intensity of incident radiation depends on the number of photons falling per unit area per unit time for a given frequency.
(iii) In photon electron collision, the total energy and momentum remain constant.
Einstein’s photoelectric equation is
Question: Consider a metal exposed to light of wavelength 600 nm. The maximum energy of the electron doubles when light of wavelength 400 nm is used. Find the work function in eV.
Answer:
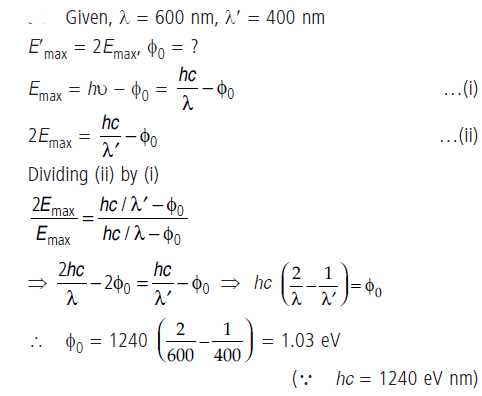
Question: A neutron beam of energy E scatters from atoms on a surface with a spacing d = 0.1 nm.
The first maximum of intensity in the reflected beam occurs at q = 30°. What is the kinetic energy E of the beam in eV?
Answer:
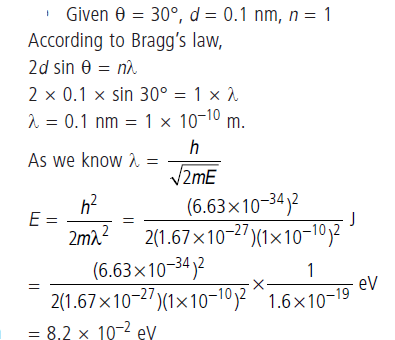
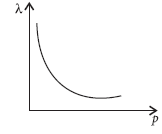
Question: (a) An electron and a proton are accelerated through the same potential. Which one of the two has (i) greater value of de-Broglie wavelength associated with it, and (ii) lesser momentum?
Justify your answer in each case.
(b) How is the momentum of a particle related with its de-Broglie wavelength? Show the variation on a graph.
Answer: For same accelerating potential, a proton and a deutron have same kinetic energy.
(a) de Broglie wavelength is given by
λ=h/P=h/√2MK=h/√2M(qV)
So, λ ∝ 1/√m
Mass of a deutron is more than that of a proton. So, proton will have greater value of de-Broglie wavelength.
(b) de-Broglie wavelength of a particle
λ=h/P
or λP=h constant
It shows a rectangular hyperbola.
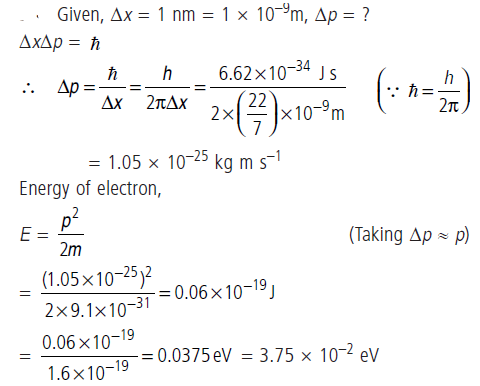
Question: Assuming an electron is confined to a 1 nm wide region, find the uncertainty in momentum using Heisenberg Uncertainty principle. You can assume the uncertainty in position Dx as 1 nm.
Assuming p ≃ Δp, find the energy of the electron in electron volts.
Answer:
Question: (a) State two important features of Einstein’s photoelectric equation.
(b) Radiation of frequency 1015 Hz is incident on two photosensitive surfaces P and Q.
There is no photoemission from surface P.
Photoemission occurs from surface Q but photoelectrons have zero kinetic energy.
Explain these observations and find the value of work function for surface Q.
Answer: (a) Two features of Einstein’s photoelectric equation: (a) Below threshold frequency uo corresponding to Wo, no emission of photoelectrons takes place. (b) As the number of photons in light depend on its intensity, and one photon liberates one photo electron. So number of emitted photoelectrons depend only on the intensity of incident light for a given frequency. (b) Below threshold frequency no emission takes place. As there is no photoemission from surface P i.e., the frequency of incident radiation is less than the threshold frequency for surface P. From surface Q photoemission is possible i.e., the frequency of incident radiation is equal or greater than threshold frequency. As the kinetic energy of photo electrons is zero i.e., the energy of incident radiation is just sufficient to pull out the electron from the surface Q.
Work function for surface Q, WQ= hu.
As K.E. = 0 ; u = u0 = 1015 Hz
WQ = 6.6 × 10–34 × 1015 = 6.6 × 10–19 J = 4.125 eV
Long Answer Type Questions
Question: Explain how Einstein’s photoelectric equation is used to describe photoelectric effect satisfactorily.
Answer: Einstein’s photoelectric equation is given below.
hν =1/2 mv2Max +W0
where u = frequency of incident radiation
1/2 mv2max = maximum kinetic energy of an emitted electron W0 = work function of the target metal Three salient features observed are
(i) Below threshold frequency u0 corresponding to W0, no emission of photoelectrons takes place.
(ii) As energy of a photon depends on the frequency of light, so the maximum kinetic energy with which photoelectron is emitted depends only on the energy of photon or on the frequency of incident radiation.
(iii) For a given frequency of incident radiation, intensity of light depends on the number of photons per unit area per unit time and one photon liberates one photoelectron, so number of photoelectrons emitted depend only on its intensity.
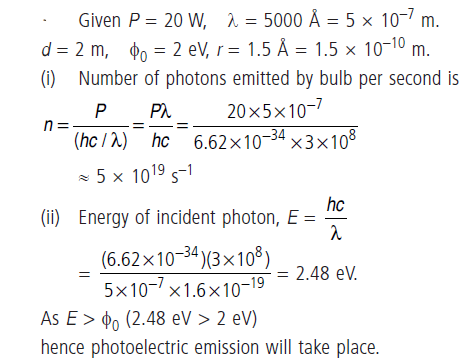
Question: Consider a 20 W bulb emitting light of wavelength of 5000 Å and shining on a metal surface kept at a distance 2 m. Assume that the metal surface has work function of 2 eV and that each atom on the metal surface can be treated as a circular disk of radius 1.5 Å. (i) Estimate no. of photons emitted by the bulb per second. [Assume no other losses] (ii) Will there be photoelectric emission? (iii) How much time would be required by the atomic disk to receive energy equal to work function (2 eV)? (iv) How many photons would atomic disk receive within time duration calculated in (iii) above? (v) Can you explain how photoelectric effect was observed instantaneously?
Answer:

Case Based MCQs
Wave Theory of Light
According to wave theory, the light of any frequency can emit electrons from metallic surface provided the intensity of light be sufficient to provided necessary energy for emission of electrons, but according to experimental observations, the light of frequency less than threshold frequency can not emit electrons; whatever be the intensity of incident light. Einstein also proposed that electromagnetic radiation is quantised.
If photoelectrons are ejected from a surface when light of wavelengthλ1 = 550 nm is incident on it. The stopping potential for such electrons is V s = 0.19 V. Suppose the radiation of wavelength λ2 = 190 nm is incident on the surface.
Question: In photoelectric effect, electrons are ejected from metals, if the incident light has a certain minimum
(a) wavelength
(b) frequency
(c) amplitude
(d) angle of incidence
Answer:
B
Question: Photoelectric effect supports quantum nature of light because
(A) there is a minimum frequency of light below which no photoelectrons are emitted.
(B) the maximum K.E. of photoelectric depends only on the frequency of light and not on its intensity.
(C) even when the metal surface is faintly illuminated, the photo electrons leave the surface immediately.
(D) electric charge of the photoelectrons is quantized.
(a) A, B, C
(b) B, C
(c) C, D
(d) A, D, C
Answer:
A
Question: Calculate the work function of the surface.
(a) 3.75
(b) 2.07
(c) 4.20
(d) 3.60
Answer:
B
Question: Calculate the stopping potential Vs2 of surface.
(a) 4.47
(b) 3.16
(c) 2.76
(d) 5.28
Answer:
A
Question: Calculate the threshold frequency for the surface.
(a) 500 × 1012 Hz
(b) 480 × 1013 Hz
(c) 520 × 1011 Hz
(d) 460 × 1013 Hz
Answer:
A
Hertz Observations
To study photoelectric effect, an emitting electrode C of a photosensitive material is kept at negative potential and collecting electrode A is kept at positive potential in an evacuated tube. When light of sufficiently high frequency falls on emitting electrode, photoelectrons are emitted which travel directly to collecting electrode and hence an electric current called photoelectric current starts flowing in the circuit, which is directly proportional to the number of photoelectrons emitted by emitting electrode
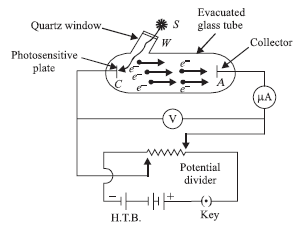
While demonstrating the existence of electromagnetic waves, Hertz found that high voltage sparks passed across the metal electrodes of the detector loop more easily when the cathode was illuminated by ultraviolet light from an arc lamp. The ultraviolet light falling on the metal surface caused the emission of negatively charged particles, which are now known to be electrons, into the surrounding space and hence enhanced the high voltage sparks.
Question: Cathode rays consists of
(a) photons
(b) electrons
(c) pistons
(d) a-particles.
Answer:
B
Question: Cathode rays were discovered by
(a) Maxwell Clerk James
(b) Heinrich Hertz
(c) William Crookes
(d) J. J. Thomson C
Answer:
C
Question: Who discovered the charge on an electron for the frist time?
(a) Millikan
(b) Thomson
(c) Kelvin
(d) Coulomb
Answer:
A



