Please refer to Assignments Class 11 Chemistry Hydrocarbons Chapter 13 with solved questions and answers. We have provided Class 11 Chemistry Assignments for all chapters on our website. These problems and solutions for Chapter 13 Hydrocarbons Class 11 Chemistry have been prepared as per the latest syllabus and books issued for the current academic year. Learn these solved important questions to get more marks in your class tests and examinations.
Hydrocarbons Assignments Class 11 Chemistry
Question. Phenyl magnesium bromide reacts with methanol to give
(a) A mixture of Benzene and Mg(OME) Br
(b) A mixture of Propane and Mg(OME) Br
(c) A mixture of Methane and Mg(OME) Br
(d) All of the above
Answer
A
Question. Aromatic compounds burn with a sooty flame because?
(a) They have a ring structure of carbon atoms
(b) They have a relatively high percentage of hydrogen
(c) They have a relatively high percentage of carbon
(d) They resist reaction with oxygen of air
Answer
C
Question. Propyne on polymerization yields
(a) Mesitylene
(b) Benzene
(c) Ethyl benzene
(d) Propyl benzene.
Answer
A
Question. Reaction of HBr with propene in the presence of peroxide gives:
(a) 3 – Bromo propane
(b) Allyl bromide
(c) n – Propyl bromide
(d) Isoproyl bromide
Answer
C
Question. Which of the following compounds will exhibit geometrical isomerism?
(a) 1 – Phenyl – 2 – butene
(b) 3 – Phenyl – 1 – butene
(c) 2 – Phenyl – 1 butene
(d) 1, 1 – Diphenyl – propene.
Answer
A
Question. The angle strain in cyclobutane is
(a) 24°44
(b) 29°16
(c) 19°22
(d) 9°44
Answer
D
Question. A gas decolourised by KMno4 solution but gives no precipitate with ammoniacal cuprous chloride is
(a) Ethene
(b) Propane
(c) Propene
(d) Methane
Answer
A
Question. The lowest alkene, that is capable of exhibiting geometrical isomerism is
(a) Ethene
(b) But – 1- ene
(c) But – 2 – ene
(d) Propene
Answer
C
Question. Ethyl benzene cannot be prepared by _____.
(a) Wurtz Reaction
(b) Wurtz Fittig reaction
(c) Clemmensen Reduction
(d) Carbon
Answer
A
Question. The lowest alkene, that is capable of exhibiting geometrical isomerism is
(a) Ethene
(b) But – 1- ene
(c) But – 2 – ene
(d) Propene.
Answer
C
Question. In which of the following compounds only primary carbon atoms are present?
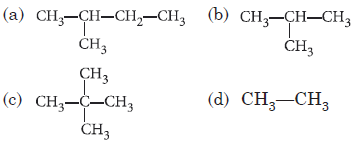
Answer
D
Question. In the following structures, which two forms are staggered conformation of ethane?
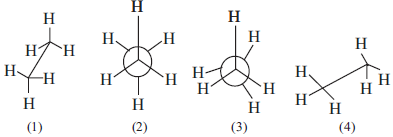
(a) 1 and 4
(b) 2 and 3
(c) 1 and 2
(d) 1 and 3
Answer
C
Question. The numbers of s and p-bonds present in 1, 3-butadiene are respectively
(a) 9 and 2
(b) 8 and 2
(c) 9 and 3
(d) 9 and 1
Answer
A
Question. Arrange the following hydrogen halides in order of their decreasing reactivity with propene.
(a) HCl > HBr > HI
(b) HBr > HI > HCl
(c) HI > HBr > HCl
(d) HCl > HI > HBr
Answer
C
Question. Arrange the following alkyl halides in decreasing order of the rate of β-elimination reaction with alcoholic KOH.

Answer
D
Question. The reaction of HBr with CH3C(CH3) == CH2 in the presence of peroxide will give
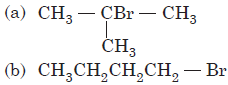
Answer
C
Question. Electrolysis of an aqueous solution of sodium ethanoate gives
(a) methane
(b) ethane
(c) butane
(d) methyl ethanoate.
Answer
B
Question. Benzene is obtained when
(a) acetylene is passed through red hot iron tube
(b) benzenesulphonic acid is treated with superheated steam
(c) both (a) and (b)
(d) none of these.
Answer
C
Question. IUPAC name of
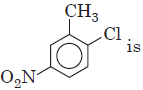
is
(a) 1-chloro-2-methyl-4-nitrobenzene
(b) 2-chloro-1-methyl-5-nitrobenzene
(c) 1-nitro-1-methyl-4-nitrobenzene
(d) 2-methyl-1-chloro-4-nitrobenzene.
Answer
A
Question. The IUPAC name of the compound having the formula CH ≡ C – CH == CH2 is
(a) 1-butyn-2-ene
(b) but-1-yn-3-ene
(c) 1-buten-3-yne
(d) 3-buten-1-yne
Answer
C
Question. The molecule having net dipole moment is
(a) 2,2-dimethylpropane
(b) trans-pent-2-ene
(c) trans-but-2-ene
(d) 2, 2, 3, 3-tetramethylbutane.
Answer
B
Question. Arrange the following in decreasing order of their boiling points.
(I) n-Butane
(II) 2-Methylbutane
(III) n-Pentane
(IV) 2,2-Dimethylpropane
(a) I > II > III > IV
(b) II > III > IV > I
(c) IV > III > II > I
(d) III > II > IV > I
Answer
D
Question. Mark the correct decreasing order of stability.
(a) Aromatic > non-aromatic > anti-aromatic
(b) Aromatic > anti-aromatic > non-aromatic
(c) Non-aromatic > anti-aromatic > aromatic
(d) Anti-aromatic > non-aromatic > aromatic
Answer
A
Question. Which of the following molecules represents the order of hybridisation sp2, sp2, sp, sp from left to right atoms?
(a) HC ≡ C — C ≡ CH
(b) CH2 == CH — C ≡ CH
(c) CH2 == CH — CH == CH2
(d) CH3 — CH == CH — CH3
Answer
B
Question. Anti-Markownikoff addition of HBr is not observed in
(a) propene
(b) 1-butene
(c) 2-butene
(d) 2-pentene
Answer
C
Question. The alkene that exhibits geometrical isomerism is
(a) propene
(b) 2-methylpropene
(c) 2-butene
(d) 2-methyl-2-butene.
Answer
C
Question. The dihedral angle HCH in staggered conformation of C2H6 is
(a) 120°
(b) 60°
(c) 0°
(d) 90°
Answer
B
Assertion & Reasoning Based MCQs :
A statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices.
(a) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(b) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(c) Assertion is correct statement but reason is wrong statement.
(d) Assertion is wrong statement but reason is correct statement.
Question. Assertion : Boiling point of alkanes increases with increase in molecular weight.
Reason : van der Waal’s forces increase with increase in molecular weight.
Answer
A
Question. Assertion : Saturated hydrocarbons are chemically less reactive.
Reason : All isomeric paraffins have same parent name.
Answer
B
Question. Assertion : Methane cannot be obtained by Wurtz reaction.
Reason : Wurtz reaction leads to the formation of symmetrical alkane having an even number of carbon atoms.
Answer
A
Question. Assertion : HC ≡ C– is more stable than H2C = C–.
Reason : HC ≡ C– has more s-character than H2C = C–.
Answer
A
Question. Assertion : Nitrobenzene does not undergo Friedel Crafts reaction.
Reason : Nitrobenzene is a m-director.
Answer
B
Question. Assertion : Sodium acetate on Kolbe’s electrolysis gives methane.
Reason : Methyl free radical is formed at anode.
Answer
D
Question. Assertion : Trans-pent-2-ene is polar but trans-but-2-ene is non-polar.
Reason : The polarity of cis-isomer is more than trans which are either non-polar or less polar.
Answer
B
Question. Assertion : Benzene on heating with conc. H2SO4 gives benzenesulphonic acid which when heated with superheated steam under pressure gives benzene.
Reason : Sulphonation is a reversible process.
Answer
A
Question. Assertion : Acetylene is acidic in nature.
Reason : Acetylene is sp hybridised.
Answer
B
Question. Assertion : All the hydrogen atoms in CH2 = C =nCH2 are attached to sp2 hybridised carbon atom.
Reason : All the carbon atoms in its are sp2 hybridized.
Answer
C

