Please refer to Assignments Class 10 Science Metals and Non-Metals Chapter 3 with solved questions and answers. We have provided Class 10 Science Assignments for all chapters on our website. These problems and solutions for Chapter 3 Metals and Non-Metals Class 10 Science have been prepared as per the latest syllabus and books issued for the current academic year. Learn these solved important questions to get more marks in your class tests and examinations.
Metals and Non-Metals Assignments Class 10 Science
Question. The reaction of metal X with Fe2O3 is highly exothermic and is used to join railway tracks. Identify metal X.
Write the chemical equation for the reaction.
Answer : X is Al.
2Al + Fe2O3 → Al2O3 + 2Fe
Question. (a) In electrolytic refining of impure copper metal, what are used as cathode and anode?
(b) Show the formation of HgCl2 from magnesium and chlorine atoms.
Answer :
(a) Pure copper is used as the cathode, impure copper is used as the anode.
(b)

Question. Write one example of each of
a. a metal which is so soft, that it can be cut with a knife and a non-metal which is the hardest known substance.
b. a metal and a non-metal which exist as liquid at room temperature.
Answer :
a. Sodium is so soft that it can be easily cut with a knife.
b. Diamond is the allotrope of carbon, which is the hardest known substance and a non-metal.
c. Mercury is a metal and Bromine is a nonmetal which are found in liquid state at room temperature.
Question. Mention the name of metals for the following:
a. Two metals which are alloyed with iron to make stainless steel.
b. Two metals which are used to make jewellery.
Answer :
a. Nickel and chromium.
b. Gold and platinum
Question. Zinc does not evolve hydrogen gas on reacting with HNO3. Why?
Answer : Dilute HNO3 is an oxidising agent, therefore it does not liberate H2 with zinc. It oxidises N and liberates NO gas (Nitrogen monoxide).
Question. Write the electron dot structure for sodium and chlorine atoms. How do these atoms form a chemical bond? Name the type of bond so formed. Why does a compound so formed have high melting point?
Answer : (Image 91), are electron dot structure of Na and chlorine atoms. Sodium can lose an electron and Cl gains that electron and they form an ionic bond.
It has high melting point due to strong forces of attraction between Na+ and Cl- ions.
Question. Give reason for the following:
a. Metals can be given different shapes according to our needs.
b. Hydrogen is not evolved when a metal reacts with nitric acid.
Answer :
a. Metals are malleable, therefore they can be given different shapes.
b. It is because HNO3(dil.) is a good oxidising agent.
Question. Why is sodium chloride soluble in water but not in kerosene?
Answer : Sodium chloride is the ionic compound and it form ions in water, therefore it is soluble in water whereas kerosene is a non-polar covalent compound, NaCl does not form ions in kerosene, therefore, it is insoluble in it.
Question. a. Why do aluminium sheets not corrode easily?
b. Why is copper vessel corroded with a green coating in rainy season?
Answer :
a. Aluminium sheets are covered with an oxide layer which makes it passive.
b. It is due to the formation of basic copper carbonate, CuCO3.Cu(OH)2.
Question. A compound Z is formed by transfer of electrons from the metal X to a non-metal Y, Identify the type of bond formed in the compound. List three properties of compound formed by such type of bonds.
Answer :
The bond formed is ionic bond:
a. The compound will have high melting and boiling point.
b. It will be soluble in water.
c. It will conduct electricity in molten state as well as in aqueous solution.
Question. Give reason for the following:
a. School bells are made up of metals.
b. Electrical wires are made up of copper.
Answer :
a. Metals are sonorous i.e., produce sound when struck with a hard substance.
b. Copper is a good conductor of electricity and is highly ductile.
Question. Out of the two metals P and Q, P is less reactive than Q. Suggest an activity to arrange these metals in the order of decreasing reactivity. Support your answer with a suitable chemical equation.
Answer : Add Q in the salt solution of P. If Q is able to displace P’ from its salt solution, then it shows P is less reactive than Q
Q + PR → QR + P
Question. Define alloy. How an is alloy prepared?
Answer : Alloy is an homogeneous mixture of two or more metals. One of them can be a non-metal also. Alloys are made by melting two metals together and then cooling it.
Question. In one of the methods of rust prevention, iron is not coated with anything. Name that method and define it.
Answer : The method is alloy formation, by adding a suitable metal or a non-metal.
Alloy is. a homogeneous mixture of two or more metals. One of them can be a non- metal also. This process is called alloying.
Question. Give reasons for the following:
a. Platinum, gold and silver are used for making jewellery.
b. Sodium, potassium and lithium are stored under kerosene oil.
c. Aluminium is highly reactive metal but still used for making cooking utensils.
d. Carbonate and sulphide ores are usually converted into oxides during the process of extraction.
Answer :
a. It is because they are lustrous metals.
b. These are highly reactive metals.
c. It forms an oxide layer on its surface which makes it passive.
d. It is easier to reduce a metal oxide than sulphides and carbonates.
Question. Differentiate between roasting and calcination giving examples.
Answer :
| Roasting | Calcination | |
| It is done in the presence of oxygen. | It is done in the absence of oxygen. | |
| It is done with sulphide ores eg. 2ZnS + 3O2 → 2ZnO + 2SO2 | It is done with carbonate ores e.g. FeCO3 → HeatFeO(s) + CO2 (g) |
Question. Name the ore of mercury. How mercury is extracted from its ore?
Answer :
Cinnabar (HgS) is the ore of mercury.
Roasting:
HgS(s) + O2(g) → 2Hg(l) + SO2(g)
Cinnabar, on roasting gives Mercury and sulphur dioxide. Mercury can be purified by distillation process.
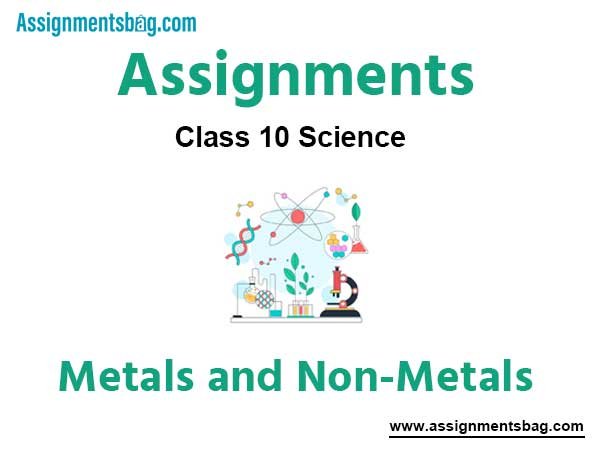
Question. How is copper obtained from Cu2S? Give reactions.
Answer :
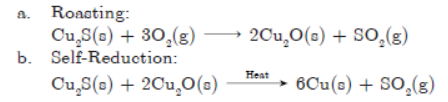
Question. Name the following:
a. A metal which is preserved in kerosene.
b. A lustrous coloured non-metal.
c. A metal which melts when kept on palm.
d. A metal which is a poor conductor of heat.
Answer :
a. Sodium or Potassium
b. Iodine
c. Gallium
d. Lead
Question. Elements magnesium and oxygen respectively belong to group 2 and group 16 of the Modern Periodic Table. If the atomic numbers of magnesium and oxygen are 12 and 8 respectively, draw their electronic configurations and show the process of formation of their compound by transfer of electrons.
Answer :
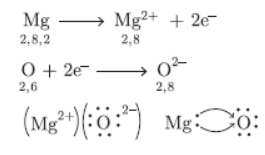
Question. Write a balanced chemical equation for representing the chemical reaction between manganese dioxide and aluminium powder. What happens if manganese powder is heated with aluminium oxide?
Answer :
3MnO2 + 4A1 → 2Al2O3 + 3Mn
No reaction will take place if manganese powder is heated with Al2O3 because Mn is less reactive than Al.
Question. Define the term alloy. Write the constituents of Bronze.
Answer :
Alloy is a homogeneous mixture of two or more metals.
One of them can be a non- metal also.
Alloys are made by melting two metals together and then cooling it.
Question. Why do silver articles turn black and copper items turn green after sometime?
Answer : Silver turns black due to the formation of Ag2S, (Silver sulphide). Copper turns green due to formation of basic copper carbonate.
Question. Name a metal/non-metal
a. Which makes iron hard and strong?
b. Which is alloyed with other metal to make amalgam?
c. Which is used to galvanise iron articles?
d. Whose articles when exposed to air forms a black coating?
Answer :
a. Carbon makes it hard and strong. Tungsten also makes it hard and strong.
b. Mercury is alloyed with metals to form amalgam.
c. Zinc metal is used for coating over iron metal to galvanise iron.
d. Silver articles acquire black coating when left exposed to the atmosphere.
Question. Which of the following listed metals can displace zinc from its salt solution? Give reason for your answer with a chemical equation:
Copper, Lead, Magnesium, Silver
Answer :
Magnesium can displace zinc from zinc salt solution because Mg is more reactive than zinc:
Mg(s) + ZnSO4(aq) → MgSO4(aq) + Zn(s)
Question. Give reasons for the following:
a. Iron grills are frequently painted.
b. Gold ornaments retain their lustre even after several years of use.
Answer :
a. It is done to prevent them from rusting.
b. Gold is least reactive, therefore it remains lustrous even after several years.
Question. Give reasons for the following:
a. Aluminium oxide is considered has amphoteric oxide.
b. Ionic compounds conduct electricity in molten state.
Answer :
a. Al2O3 reacts with acids as well as bases, therefore it is regarded as amphoteric oxide.
b. In molten state, ions are free to move, therefore it conducts electricity.
Question. How do properties of iron change when
a. a small quantity of carbon is mixed in it?
b. nickel and chromium are mixed in it?
Answer :
a. It becomes hard and does not get rusted.
b. It becomes lustrous and malleable and so it does not get rusted.
Question. You must have seen tarnished copper vessels being cleaned with lemon or tamarind juice. Explain why these sour substances are effective in cleaning the vessels.
Answer : Copper vessel coated with a green layer of CuCO3.
Cu(OH)2 which reacts with acid present in lemon or tamarind juice and get removed it. Some substances contain acids which react with basic substances to form soluble salt which can be easily removed.
Question. What happens to potassium and sodium if they are kept in open? Why are they 3 immersed in kerosene oil?
Answer : Potassium and Sodium if kept in open, can catch the presence of moisture.
They are immersed in kerosene so that they do not react with air and H2O present in the atmosphere.
Question. Why hydrogen gas is not evolved when a metal reacts with nitric acid?
Answer : Nitric acid is an oxidising agent, therefore H2(g) is not evolved on reaction with metal. Dilute HNO3 mostly gets reduced to NO in this process.
Question. Why is iron galvanised with zinc? Can it be galvanised with copper? If not, why?
Answer : Iron is galvanised by zinc because zinc is more reactive than Fe. Iron cannot be galvanised by copper because copper is less reactive than iron and therefore iron itself gets corroded when coated with copper.
Question. Name two metals which can be used to reduce metal oxides to metals.
Answer :
Aluminium and Magnesium
2Al + Fe2O3 → Al2O3 + 2Fe
3Mg + Fe2O3 → 3MgO + 2Fe
Question. Write four important purposes of making alloys.
Answer :
a. It is done to increase the hardness of the substance.
b. It is done so as to increase the resistance towards corrosion.
c. It helps to modify reactivity.
d. It lowers the melting point.
Question. State four general properties of ionic compounds.
Answer :
a. They are soluble in water.
b. They are hard but brittle solids.
c. They conduct electricity in solid state and in their aqueous solution.
d. They have high melting and boiling points.
Question. The way, metals like sodium, magnesium and iron react with air and water is an indication of their relative positions in the ‘reactivity series’. Is this statement true? Justify your answer with example.
Answer :
Yes, Na reacts vigorously with cold water because it is highly reactive. Magnesium reacts with hard water because it is less reactive than sodium. Iron reacts only with steam because it is less reactive than Mg:
2Na(s) + MgO(s) → Na2O(s) + Mg
3Mg + Fe2O3 → 3MgO + 2Fe.
Question. What are amphoteric oxides? Give two examples.
Answer : Those oxides which react with acids as well as bases are called amphoteric oxides e.g., Al2O3 and ZnO.
Question. X + YSO4 → XSO4 + Y
Y + XSO4 → No reaction
Out of the two elements, X and Y, which is more reactive and why?
Answer : X is more reactive than Y because it is displacing Y from its salt solution.
Question. What is an alloy? State the constituents of solder.
Which property of solder makes it suitable for welding electrical wires?
Answer :
Alloy is a homogeneous mixture of two or more metals.
One of them can be a non- metal also.
Alloys are made by melting two metals together and then cooling the mould of two metals being mixed that forms the alloy.
Solder is an alloy of lead and tin. It has low melting point, therefore it is used for welding purposes.
Question. Using the electronic configuration, explain how magnesium atom combines with oxygen atom to form magnesium oxide by transfer of electrons.
Answer :
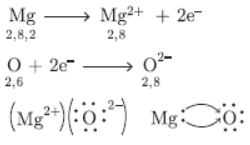
Question. A metal A which is used in thermite process, when heated with oxygen gives an oxide B which is amphoteric in nature. Identify A and B. Write down the reactions of oxide of B with HCl and NaOH.
Answer :
A is Aluminium
2Al + 3O2 →Heat Al2O3(S)
Al2O3 is an amphoteric oxide:
Al2O3 + 2NaOH → 2NaAlO2 + H2O
Al2O3 + 6HCl →2AlCl3 + 3H2O
Question. A substance X which is an oxide of metal is intensively used in cement industry. The element is present in our bones also. On treatment with water, it forms a solution which turns red litmus blue. Identify X and also write the chemical reactions involved.
Answer :
X is calcium. Its oxide, CaO is used in cement industry.
Calcium is also present in bones.
Ca(s) + 2H2O(l) → Ca(OH)2(aq) + H2(g)
Calcium hydroxide turns red litmus blue.
Question. Write chemical equations that shows aluminium oxide reacts with acid as well base.
Answer : Al2O3 + 2NaOH → 2NaAlO2 + H2O
Al2O3 + 3H2SO4 → Al2(SO4)3 + 3H2O
Question. Explain, why calcium metal after reacting with water starts floating on its surface. Write the chemical equation for the reaction.
Answer :
Calcium reacts with water to form Ca(OH)2 and H2 gas.
Ca(s) + 2H2O(l) → Ca(OH)2(aq) + H2(g)
The bubbles of hydrogen gas sticks to the surface of calcium and therefore it floats over water.
Question. When a metal X is reacted with cold water, it gives a basic compound Y with molecular formula XOH (Molecular mass 40) and liberates a gas Z which easily catches fire. Identify X, Y, Z.
Answer : X is Na (Sodium).
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(s)
Molecular weight of NaOH = 40 g mol-1. Y is NaOH and Z is H2 gas which easily catches fire.
Question. Why is carbon not used for reducing aluminium oxide to obtain Al?
or
Metals placed high in reactivity series cannot be obtained from their compounds by heating with carbon?
Answer :
It is because Al itself is a strong reducing agent, therefore it cannot be reduced by carbon.
Question. If a strip of aluminium with scratched clean surface is dipped into an aqueous solution of copper sulphate for a little time, the surface of the strip becomes brownish. What is the reason for this? Write the balanced chemical equation for this.
Answer : Aluminium is more reactive than copper,
2Al(s) + 3CuSO4(aq) → Al2(SO4)3(aq) + 3Cu(s)
Question. A copper plate was dipped into a solution of silver nitrate. After sometime a black layer was observed on the surface of copper plate. State the reason for it and write chemical equation for the reaction involved.
Answer : Copper is more reactive than Ag, therefore it can displace Ag from AgNO3 solution:
Cu(s) + 2AgNO3(aq) → Cu(NO3)2(aq) + 2Ag(s)
Question. Give reason:
a. Carbonate and sulphide ores are usually converted into oxides during the process of extraction of metals.
b. Ionic compounds generally have high melting point.
c. Hydrogen is not a metal but has been assigned a place in the reactivity services of metals.
d. The galvanised iron article is protected against rusting even if the zinc layer is broken.
Answer :
a. It is easier to reduce an oxide than sulphide and carbonate ores.
b. It is due to strong forces of attraction between positive and negative ions.
c. Hydrogen form H+ ions like metals, therefore it is included in the activity series.
d. Zinc is more reactive than iron, therefore it will get easily oxidised in preference to Fe and hence protect iron from rusting even if zinc coating is broken.
Short Answer Questions
Question. a. Write the steps involved in the extraction of pure metals in the middle of the activity series from their carbonate ores.
b. How is copper extracted from its sulphide ore?
Explain the various steps supported by chemical equations. Draw a labelled diagram for the electrolytic refining of copper.
Answer :
a. Following steps are involved in the
i. Hydraulic washing: The carbonate ores is washed with stream of water to remove earthly impurities.
ii. Calcination: Carbonate ores is heated strongly in the absence of air to form oxides e.g.,
ZnCO3 →Heat ZnO + CO
iii. Reduction: ZnO is reduced with carbon to get Zn:
ZnO + C → Zn + CO
iv. Electrolytic refining: Impure zinc is made as anode, pure Zn is taken as cathode, acidified ZnSO4 of electrolyte’is used to get pure metal:
At anode: Zn → Zn2+ + 2e-
(Impure)
At cathode: Zn2+ + 2e- → Zn(s)
(Pure)
b. Copper sulphite is concentrated by froth floation process.
i. 2Cu2S + 3O2 → 2Cu2O + 2SO2
ii. Reduction: Cu2S + 2Cu2O → 6Cu + SO2
iii. Electrolytic refining:
At cathode: Cu2+ + 2e- → Cu
(pure)
At anode: Cu → Cu2+ + 2e-
(Impure)
Acidified CuSO4 is taken as electrolyte.

Question. Explain the following statements:
a. Most metal oxides are insoluble in water but some of these dissolve in water. What are these oxides and their solution in water called?
b. At ordinary temperature the surface of metals such as magnesium, aluminium, zinc etc. is covered with a thin layer. What is the composition of this layer? State its importance.
c. Some alkali metals can be cut with knife.
Answer :
a. These oxides are basic in nature and their solution are called alkalies.
b. The composition of this layer is metal oxide. It prevents the metal from corrosion.
c. Sodium can be cut with a knife because it is a very soft metal.
Question. Distinguish between the following:
a. Electrolytic reduction and electrolytic refining.
b. Mineral and ore.
c. Alloys and amalgams
Answer : (Image 104)
Question. Write one example of each of the following:
a. Most malleable and most ductile metal.
b. The best conductor of heat and poorest conductor of heat.
c. ‘A metal with highest melting point and a metal with lowest melting point.
Answer :
a. Gold is most malleable and ductile.
b. Copper is the best conductor and lead is a poor conductor of heat copper.
c. Tungsten has highest melting point, mercury has lowest melting point.
Question. Aluminium oxide and zinc oxide react with both acids and bases to produce salt and water. What are these oxides called? Write chemical equations in each case.
Answer : These are called amphoteric oxides:
Al2O3 + 2NaOH → 2NaAlO2 + H2O
ZnO + 2NaOH → Na2ZnO2 + H2O
ZnO + 2HCl → ZnCl2 + H2O
Al2O3 + 6HCl → 2AlCl3 + 3H2O
Question. Distinguish between the following:
a. Electrolytic reduction and electrolytic refining.
b. Mineral and ore.
c. Alloys and amalgams
Answer :
| Electrolytic reduction | Electrolytic refining |
| Metal is obtained by electrolysis of molten ore. | Metal is refined by taking impure metal as anode, pure metal as cathode and soluble salt of metal as electrolyte and by passing electric current. |
| Mineral | ore |
| Naturally occurring substances from which metal may or may not be extracted economically e.g., Mica. | Naturally occurring substances from which metal is extracted profitably e.g., Bauxite is an ore of Al. |
| Alloys | amalgams |
| Homogeneous mixture of two or more metals. One of them can be a nonmetal also e.g., steel. | Homogenous mixture of mercury with any other metals e.g., zinc amalagam. |
Question. Describe an activity to show that metals are good conductors of electricity.
Answer : Activity: To show metals are good conductors of electricity.
1. Take copper wire and insert two clips with a sample to be inserted between them, as shown in the diagram.
2. Set up the apparatus as shown in the diagram.
3. Plug the switch ON so that current starts flowing.
4. Record your observations.
5. Repeat the procedure with other metals.
Observation: The bulb glows with all metals.
Conclusion: Metals are good conductors of electricity.

Question. a. What is an alloy? How is it prepared? Give two examples of alloys.
b. Iron is not used in pure state. Give reason.
Answer :
a. Alloy is a homogeneous mixture of two or more metals. One of them can be a non-metal also.
Alloys are made by melting two metals together and then cooling it. Example: Brass and Bronze
b. Iron gets rusted, therefore it is not used in pure state.
Question. A metal P when exposed to moist air for a longer period of time, loses its shining brown surface and attains a green coating; what has happened? Identify the metal, write the name and chemical formula of green coloured compound. List two ways to prevent this process.
Answer :
It undergoes corrosion. The metal is copper.
Chemical formula of green coloured compound is CuCO3.Cu(OH)2
a. It can be prevented by coating of tin over iron.
b. It can also be prevented by coating it with paint.
Question. Give reasons only:
a. We can store copper sulphate solution in a silver vessel but not silver nitrate solution in copper vessel.
b. The reaction of zinc with dilute HNO3 does not produce H2 gas.
c. Food cans are coated with tin rather than zinc.
Answer :
a. It is because copper is more reactive than silver,
it will displace Ag from AgNO3 solution. Thus we can’t store AgNO3 in a copper container but CuSO4 can be stored in silver vessel as no reaction will take place because silver is less reactive.
b. Dilute HNO3 is an oxidising agent.
c. Tin is less reactive and less expensive than zinc.
Question. Name a metal in each case:
a. It displaces hydrogen gas on reaction with nitric acid.
b. It does react with any physical state of water.
c. It does not react with cold water or hot water, but reacts with steam.
Answer :
a. Mg and Mn
b. Cu
c. Iron
Question. (a) What is reactivity series? How does the reactivity series of metals help in predicting the relative activity of various metals?
(b) Suggest different chemical processes used for obtaining a metal from its oxides of metals in the middle of the reactivity series and metals at the top of the reactivity series. Support your answer with one example each.
Answer : (a) The series in which metals are arranged in decreasing order of reactivity is called activity series of metals. The metal at the top is most reactive, followed by less reactive metal and so on.
The metal at the bottom of activity series is least reactive.
(b) Metals at the top of activity series are obtained by electrolytic reduction e.g.,
NaCl → Na++ Cl-
(Molten)
At cathode :
Na++ e– → Na
At anode:
Cl–e– → Cl
Cl + Cl → Cl2
Metals in the middle of the reactivity series are obtained by reduction with A1 e.g.,
Cr2O3 + 2Al → Al2O3 + 2Cr
Question. State three reasons for the following facts:
a. Sulphur is a non-metal.
b. Magnesium is a metal.
One of the reason must be supported with a chemical equation.
Answer :
a. Sulphur is a non-metal because it reacts with O2 to form SO2 which is an acidic oxide: S + O2 → SO2 Magnesium is a metal which reacts with O2 to form basic oxide:
2Mg + O2 → 2MgO
b. Sulphur is not malleable and ductile, magnesium is malleable and ductile.
c. Sulphur does not conduct electricity whereas magnesium conducts electricity.
Question. What is cinnabar? How is metal extracted from cinnabar? Explain briefly.
Answer :
Cinnabar (HgS) is an ore of mercury.
Roasting: HgS(s) + O2(g) → 2Hg(l) + SO2(g)
Cinnabar, on roasting gives Mercury and sulphur
dioxide. Mercury can be purified by the process of distillation.
Question. a Write the electron dot structure of Potassium (19) and Chlorine (17).
b. Show the formation of KCl by transfer of electrons.
c. Name the ions present in the compound, KCl.
Answer :
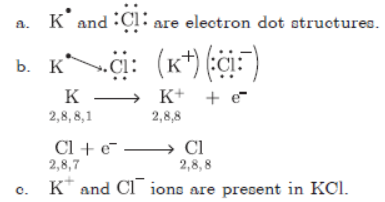
Question. a. Write the electron-dot structure of calcium and sulphur.
b. Show the formation of CaS by transfer of electrons.
c. Name the ions present in the compound, CaS.
Atomic number of Ca = 20, S = 16.
Answer :

Question. Give one most suitable word for the following statements:
a. Metal oxides which show basic as well as acidic behaviour.
b. Some metals produce a sound on striking with a hard surface.
c. Iodine, shining non-metal.
Answer :
a. Amploteric oxide,
b. Sonorous,
c. Lustrous.
Question. Name the constituent elements of alloys:
a. Brass,
b. Bronze,
c. Solder.
Mention one use of each alloy.
Answer :
a. Brass is made up Cu and Zn. It is used for making decorative articles.
b. Bronze is made up of Cu and Sn. It is used for making statues, medals.
c. Solder it is an alloy of Pb and Sn. It is used for soldering purposes.
Question. State the property utilised in the following:
a. Graphite in making electrodes.
b. Electric wires are coated with polyvinyl chloride (PVC) or rubber like materials.
c. Metal alloys are used for making bells and strings of music instruments.
Answer :
a. Graphite is a good conductor of electricity.
b. PVC is an insulator, protects us from electric current.
c. Alloys are stronger and more sonorous than metals and used for bells and musical instruments. They do not get rusted.
Question. Write chemical equations for the reactions taking place when:
a. Iron reacts with steam.
b. Magnesium (Mg) reacts with dilute HCl.
c. Copper is heated in air.
Answer :
a. 3Fe(s) + 4H2O(g) Heat Fe3O4(s) + 4H2(g)
b. Mg(s) + 2HCl(dil.) → MgCl2(aq) + H2(g)
c. 2Cu(s) + O2(g) → 2CuO(s)
Question. How metals can be differentiated from non-metals on the basis of any of it three chemical roperties?
Answer :
| Metal | Non-metals | |
| 1. | Metallic oxides are basic in nature. | Non-metallic oxides are acidic in nature |
| 2. | Most of metals liberate H2 gas with dilute acids. | Non-metals do not liberate H2 gas with dilute acids. |
| 3. | Metal hydroxides, bases turn red litmus blue. | Non metallic oxides form acid in aqueous solution, which turns blue litmus red. |
Question. Suggest a method of reduction for the following metals during metallurgical processes:
a. Metal A which is one of the last, second or at the third position in the reactivity series.
b. Metal B which gives vigorous reaction even with water and air.
c. Metal C which is kept in the middle of the activity series.
Answer :
a. Reduction with carbon.
b. Electrolytic reduction.
c. Reduction with Al.
Question. a. Explain the formation of ionic compound CaO with its electron dot structure. Atomic number of Ca = 20, O = 8.
b. Name the constituents of bronze.
Answer :
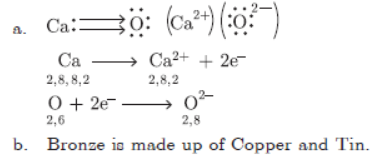
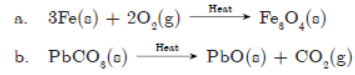
Question. Write chemical equations for the reactions taking place when:
a. Iron is strongly heated with air.
b. Lead carbonate is calcined.
c. Chromium oxide (Cr2O3) is heated with aluminium powder.
Answer :

Question. An ore on heating in air produces sulphur dioxide.
Which process would you suggest for its concentration?
Describe briefly any two steps involved in the conversion of the concentrated ore into the related metal.
Answer :
It is concentrated by using froth floatation process.
a. Roasting: The ore is heated in the presence of oxygen to form oxide e.g.,
2ZnS + 3O2 → 2ZnO + SO2
b. Reduction: ZnS is reduced by ZnO to get Zn and SO2. Carbon can also be used to reduce ZnO to
Zn:
ZnS + 2ZnO → 3Zn + SO2
ZnO + C → Zn + CO
Question. What is meant by rusting? With labelled diagrams, describe an activity to find out the conditions in which rusting takes place.
Answer : The process in which iron reacts with oxygen in the presence of moisture to form brown layer on its surface is called rusting.

Question. Observe the two test tubes A and B as shown in the diagram given below and answer the following questions :
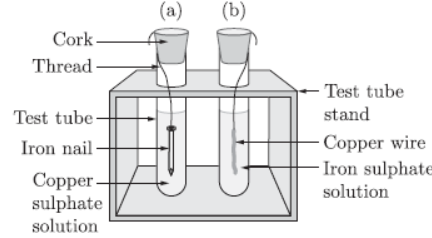
a. In which test tube will the reaction take place?
b. Write a balanced chemical equation for the reaction.
c. Name the type of reaction.
Answer :
a. In the first test tube reaction will take place.
b. Fe(s) + CuSO4(aq) → FeSO4(aq) + Cu(s)
c. Displacement reaction.
Question. A, B and C are three elements which undergo chemical reactions according to the following equations:
A2O3 + 2B → B2O3 + 2A ,
3CSO4 + 2B → B2(SO4)3 + 3C
3CO + 2A → A2O3 + 3C
a. Which element is most reactive?
b. Which element is the least reactive?
c. What type of reactions is listed above?
Answer :
a. B is most reactive.
b. C is least reactive.
c. Displacement reactions.
Question. A metal forms an oxide having formula M2O3. It dissolves both in dilute sulphuric acid as well as dilute sodium hydroxide solution. Identify the metal and write the equations for the reactions involved.
Answer :
The metal is Al. Its oxide is Al2O3.
Al2O3 + 2NaOH → NaAlO2 + H2O
Al2O3 + 3H2SO4(aq) → Al2(SO4)3(aq) + 3H2O(l)
Question. State the conditions under which the following metals react with water. Write chemical equation for its reaction with each: (a) Na (b) Mg (c) Fe
Answer :
(a) Sodium metal reacts with cold water as:
2Na + 2H2O → 2NaOH + H2
(b) Magnesium reacts with hot water as:
Mg + 2H2O → Mg(OH)2 + H2
(c) Iron reacts with steam as:
3Fe + 4H2O → Fe3O4 + 4H2
Question. List three properties of sodium in which it differs from general physical properties of most metals.
Answer :
a. It is soft.
b. It has low melting and boiling point.
c. It is not malleable and ductile.
Question. a. Define the term ‘anode mud’. Name an electrode made of pure metal.
b. Give the reactions taking place at cathode and anode during the electrolytic refining of copper.
Answer :
a. The impurities left behind at anode after impure copper metal undergoes electrolytic refining is called anode mud.
b. At anode:
Cu(s) → Cu2+ (aq) + 2e-
(impure)
At cathode:
Cu (aq) + 2e– → Cu(s)
(Pure)
Question. Name two metals which are purified by electrolytic refining. Mention the cathode, anode and electrolyte used in the refining process. At which electrode would the pure metal be deposited?
Answer :
Cu, Zn, Al can be purified by electrolytic refining method:
At cathode, pure metal is deposited. Cathode is made up of pure metal. Anode is made up of impure metal.
Soluble salt of metal is used as electrolyte.
Question. Write the balanced chemical equation in each case:
a. Mg metal is reacted with very, little amount of dilute HNO3.
b. Aluminium powder is added to Fe2Og.
c. Zinc sulphide is roasted.
Answer :
a. Mg(s) + 2HNO3(S) → Mg(NO3)2(aq) + H2
3Mg(s) + dil.HNO3 → Mg(NO3)2 + 2NO + 4H2O
b. 2A1 + Fe2O3 → Al2O3 + 2Fe
c. 2ZnS + 3O2 → 2ZnO + 2SO2
Question. a. Show the formation of Na2O by transfer of electrons between the combining atoms.
b. Why are ionic compounds usually hard?
c. How is it that ionic compound in the solid state do not conduct electricity but they do so in the molten state?
Answer :
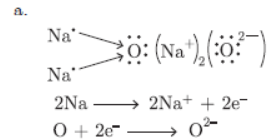
b. Ionic compounds are hard due to strong forces of attraction between oppositely charged ions.
c. In solid state, ions are not free to move but in molten state, ions are free to move.
Question. Define the terms: (a) mineral, (b) ore and (c) gangue
Answer :
a. Mineral: It is a naturally occurring substance from which metal may or may not be extracted economically e.g., Mica.
b. Ores: These are naturally occurring substances from which metal can be extracted profitably e.g., Haematite.
c. Gangue: The impurities present in the ore is called gangue e.g., SiO2.
Question. Describe ionic compounds on the basis of following properties:
a. Strong forces of attraction between positive and negative ions.
b. Solubility of compounds in water.
c. Electrical conductivity.
Answer :
a. They have high melting and boiling points.
b. They are soluble in water.
c. They conduct electricity in molten state and in aqueous solution.
Question. a. Name the metal which does not stick to the glass,
b. Name a metal which is commonly used in thermite welding.
c. What is the nature of Zinc oxide?
Answer :
a. Mercury.
b. Aluminium displaces molten iron from Fe2O3 which is used in thermite reaction for welding purpose.
c. It is amphoteric.
Long Answer Questions
Question. a. Define corrosion.
b. What is corrosion of iron called?
c. How will you recognise the phenomena of corrosion of silver?
d. Why corrosion of iron metal is a serious problem?
e. How can we prevent corrosion of iron?
Answer :
a. Corrosion: The process in which a metal react with substances present in atmosphere to form surface compounds is called corrosion.
b. Corrosion of iron is called rusting.
c. It turns black on corrosion due to the formation of Ag2S.
d. It weakens bridges and materials and a lot of iron gets wasted every year.
e. We can prevent corrosion of iron by
1. Painting,
2. Oiling and greasing,
3. Galvanisation,
4. By forming its alloys.
Question. a. Write electron dot diagrams of chlorine (At. No. 17) and calcium (At. No. 20). Show the formation of calcium chloride by transfer of electrons.
b. Identify the nature of the above compound and explain three physical properties of such a compound.
Answer :
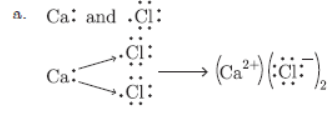
b. It is an ionic compound.
1. It is soluble in water.
2. It conducts electricity in molten state and in aqueous solution.
3. It is a hard solid.
Question. a. What type of ores are calcined? Illustrate with suitable examples.
b. In what form calcined ore is obtained and how it can be reduced? Give chemical equation for the reduction process involved for the example given by you.
c. Name any two metals used as reducing agents by displacing metals of low reactivity from their compounds.
Answer :
a. Carbonate ores are calcined e.g.,
CuCO3(s) →Heat CuO(s) + CO2(g)
b. It is obtained in oxide form. It is reduced by using a suitable reducing agent e.g.,
CuO(s) + C →Heat Cu(s) + CO(g)
c. Al and Mg.
Question. a. An ore on treatment with dilute hydrochloric acid produces brisk effervescence. What kind of ore is this? What steps will be required to obtain metal from the enriched ore?
b. Copper coin is kept immersed in silver nitrate solution for sometime. What change .will take place in the coin and in the colour of the solution?
Write a balanced chemical equation for the reaction.
Answer :
a. It is a carbonate ore.
(i) Hydraulic washing, (ii) Calcination,
(iii) Reduction, (iv) Refining.
b. The solution will become blue in colour, blackish silver metal will get deposited:
Cu(s) +2AgNO3 (aq) → Cu(NO3)2 (aq) 2Ag(s)
Question. a. Define activity series of metals. Arrange the metals: gold, copper, iron and magnesium in the order of their increasing reactivity.
b. What will you observe when:
(1) Some zinc pieces are placed in copper sulphate solution.
(2) Some silver pieces are placed into green coloured ferrous sulphate solution.
Answer :
a. The series in which metals are arranged in decreasing order of reactivity is called activity series of metals. The metal at the top is most reactive, followed by less reactive metal and so on.
The metal at the bottom is least reactive.
Au < Cu < Fe < Mg is the increasing order of reactivity.
b. (1) The solution will become colourless, reddish brown copper metal will get deposited:
Zn(s) + CuSO4(aq) → ZnSO4(aq) + Cu(s)
(2) No reaction will take place because Ag is less reactive than Fe.
Question. A metal (E) is stored under kerosene oil. When a small piece of it is left open in air, it catches fire. When the product formed is dissolved in water, it turns red litmus blue.
a. Name the metal (E).
b. Write the chemical equation for the reaction
when it is exposed to air and when the product is dissolved in water.
c. Explain the process by which metal is obtained from its molten chloride.
Answer :
a. E is sodium metal.
b. 2Na + 2H2O → 2NaOH + H2
H2 gas will catch fire.
NaOH turns red litmus blue.
4Na(s) + O2(s) → 2Na2O(s)
Na2O + H2O(aq) → 2NaOH(aq)
c. It is obtained by electrolytic reduction e.g., ‘Na’ metal is obtained by electrolysis of molten NaCl:
NaCl Electrolysis N+ + Cl–
Molten
At cathode: Na+ + e– → Na
At anode: Cl- → Cl + e–
Cl + Cl → Cl2
Question. a. Write two differences between calcination and roasting.
b. ‘No reaction takes place when granules of a solid ‘A’ is mixed with a powder of solid ‘B’. However when the mixture is heated, a reaction starts with the evolution of much heat. Product ‘C’ of the reaction settles down as a liquid metal and a solid product ‘D’ keeps on floating over the liquid ‘C’.
This reaction is sometimes used for making metals for ready use in odd places.
a. Based on this information, make assumptions about metals ‘A’ and ‘B’ and corresponding deductions about ‘C’ and ‘D’ and write a balanced chemical equation for the reaction. Include in the chemical equation about the physical states of the reactants and products, need for heating for starting the reaction and the reaction being exothermic.
b. Name two types of chemical reactions to which this reaction can belong to.
Answer :
a. Differences between calcination and roasting.
| Roasting | Calcination | |
| 1. | It is done in the presence of oxygen. | It is done in the absence of oxygen. |
| 2. | It is done with sulphide ores eg. | It is done with carbonate ores e.g. |
b. A is Al, B is Fe2O3, C is molten Iron, D is Al2O3
(Molten)
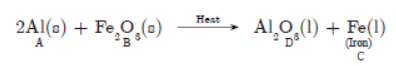
This reaction need heat to start but it is highly exothermic.
This reaction belongs to the category of:
(1) Displacement reaction,
(2) Redox reaction.
Question. Four metals A, B, C and D are added to the following aqueous solutions one by one. The observations made are tabulated below:
| Iron (II) sulphate | Copper (II) sulphate | Zinc sulphate | Silver nitrate | |
| A. | No Reaction | Reddish Brown Deposit | ………. | ………. |
| B. | Grey Deposit | ………. | No Reaction | ………. |
| C. | No Reaction | No Reaction | No Reaction | White Shining Deposit |
| D. | No Reaction | No Reaction | No Reaction | No Reaction |