VBQs Chemical Reactions And Equations Class 10 Science with solutions has been provided below for standard students. We have provided chapter wise VBQ for Class 10 Science with solutions. The following Chemical Reactions And Equations Class 10 Science value based questions with answers will come in your exams. Students should understand the concepts and learn the solved cased based VBQs provided below. This will help you to get better marks in class 10 examinations.
Chemical Reactions And Equations VBQs Class 10 Science
Objective Questions
Question. A substance which oxidises itself and reduces other is known as –
(a) oxidising agent
(b) reducing agent
(c) both of these
(d) none of these
Answer
B
Question. Which of the following is a physical change?
(a) Formation of curd from milk
(b) Ripening of fruits
(c) Getting salt from sea water
(d) Burning of wood
Answer
C
Question. The reaction in which two compounds exchange their ions to form two new compounds is –
(a) a displacement reaction
(b) a decomposition reaction
(c) an isomerization reaction
(d) a double displacement reaction
Answer
D
Question. A redox reaction is one in which-
(a) both the substance are reduced
(b) both the substance are oxidised
(c) an acid is neutralised by the base
(d) one substance is oxidised while the other is reduced
Answer
D
Question. When the gases sulphur dioxide and hydrogen sulphide mix in the presence of water, the reaction is SO2 + 2H2S → 2H2O + 3S . Here hydrogen sulphide is acting as –
(a) an oxidising agent
(b) a reducing agent
(c) a dehydrating agent
(d) a catalyst
Answer
B
Question. CuO + H2 → H2O + Cu, reaction is an example of –
(a) redox reaction
(b) synthesis reaction
(c) neutralisation
(d) analysis reaction
Answer
A
Question. In the following equations :
Na2CO3 x HCl → 2NaCl + CO2 + H2O the value of x is-
(a) 1
(b) 2
(c) 3
(d) 4
Answer
B
Question. In the equation, NaOH HNO3 NaNO3 H2O nitric acid is acting as-
(a) an oxidising agent
(b) an acid
(c) a nitrating agent
(d) a dehydrating agent
Answer
B
Question. Fe2O3 + 2Al → Al2O3 + 2Fe The above reaction is an example of a-
(a) combination reaction
(b) double displacement reaction
(c) decomposition reaction
(d) displacement reaction
Answer
D
Question. White silver chloride in sunlight turns to-
(a) grey
(b) yellow
(c) remain white
(d) red
Answer
A
Question. Black and white photography uses-
(a) decomposition of silver chloride
(b) decomposition of silver bromide
(c) both
(d) none of these
Answer
B
Question. When copper powder is heated it gets coated with-
(a) black copper oxide
(b) yellow copper oxide
(c) red copper oxide
(d) None of these
Answer
A
Question. Combination of phosphorus and oxygen is an example of –
(a) oxidation
(b) reduction
(c) rancidity
(d) None of these
Answer
A
Question. To indicate the presence of gaseous reactant or product, we use the symbol
(a) (Product) g or (Reactant) g
(b) (Product) ↑ or (Reactant) ↑
(c) (Product) ↓ or (Reactant) ↓
Answer
D
Question. When Ca(NO3)2 is heated, it gives CaO, NO2(g) and O2(g). The correct number of moles of Ca(NO3)2 , CaO, NO2(g) and O2(g) are present in the reaction are respectively
(a) 2, 1, 3, 2
(b) 2, 2, 4, 1
(c) 2, 2, 2, 1
(d) 1, 2, 4, 1
Answer
B
Question. Which of the following reaction is characterised by the yellow colour of product?
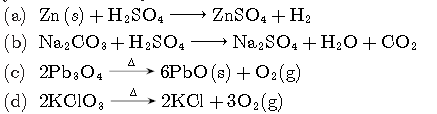
Answer
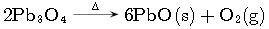
Question. Which one of the following involve a chemical reaction?
(a) Evaporation of water
(b) Storing on nitrogen gas under pressure
(c) Keeping petrol in a China dish in open
(d) Heating magnesium wire in the presence of air at high temperature
Answer
D
Question. Ethane (C2H6) on complete combustion gave CO2 and water. It shows that the results are in accordance with the law of conservation of mass. Then, the coefficient of oxygen is equal to
(a) 3
(b) 5/2
(c) 2
(d) 7/2
Answer
D
Question. A powdered salt (X) in a dry test tube was heated that evolves brown fumes of nitrogen dioxide and a yellow residue of lead oxide is also formed. The salt (X) is
(a) MgSO3
(b) Pb(NO3)2
(c) (NH4)2SO4
(d) CaCO3
Answer
B
Question. A metal ‘M’ reacts with an acid according to the equation.
M + H+ → M3+ + H2
Which of the following is correct for metal M?
(a) Calcium
(b) Aluminium
(c) Barium
(d) Potassium
Answer
C
Fill In The Blank
Question. When calcium carbonate is heated, it decomposes to from ………. and ………. gas.
Answer
calcium oxide, carbon dioxide
Answer
barium sulphate
Question. Two different atoms or groups of atoms (ions) are exchanged in ………. reactions.
Answer
double displacement
Question. Precipitation reactions produce ……… salts.
Answer
insoluble
Question. Reduction is the ………. of oxygen or gain of hydrogen.
Answer
loss
Question. The addition of oxygen to a substance is called ……….
Answer
oxidation
Question. The digestion of food in the body is an example of ……… reaction.
Answer
decomposition reaction
Question. The addition of oxygen to a substance is called ……….
Answer
oxidation
Question. When calcium carbonate is heated, it decomposes to give ………. and ……….
Answer
CaO (s) and CO2 (g)
Question. In a ………. reaction two or more substances combine to form a new single substance.
Answer
combination
Question. Reactions in which heat is given out along with the products are called ………. reactions.
Answer
exothermic
Question. When an element displaces another element from its compound, a ………. reaction occurs.
Answer
displacement
True/False
Question. A complete chemical equation represents the reactants, products and their physical states symbolically.
Answer
True
Question. The reaction between nitrogen and hydrogen to give ammonia is an example of a combination reaction.
Answer
True
Question. For word-equations, we do no need to know the formulae for the chemicals involved but in symbol equations we do.
Answer
True
Question. Action of heat on ferrous sulphate is an example of decomposition reaction.
Answer
True
Matching Questions
Direction : Each question contains statements given in two columns which have to be matched. Statements (A, B, C, D) in column I have to be matched with statements (p, q, r, s) in column II.
Question.
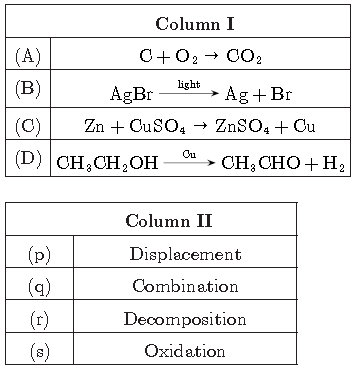
Answer
A-q, B-r, C-p, D-s
Question.
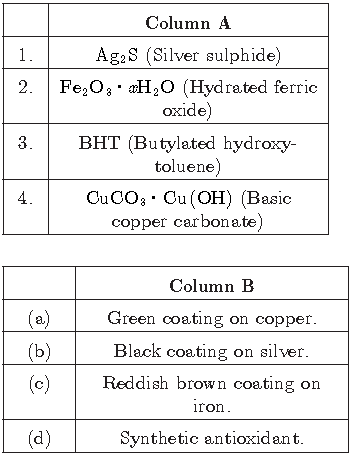
Answer
1-(b), 2-(c), 3-(d), 4-(a)
Direction : Following question has four statements (A, B, C and D) given in Column I and four statements (p, q, r and s) in Column II. Any given statement in Column I can have correct matching with one or more statement(s) given in Column II. Match the entries in column I with entries in column II.
Question. Column II gives type of reaction mention in column I, match them correctly
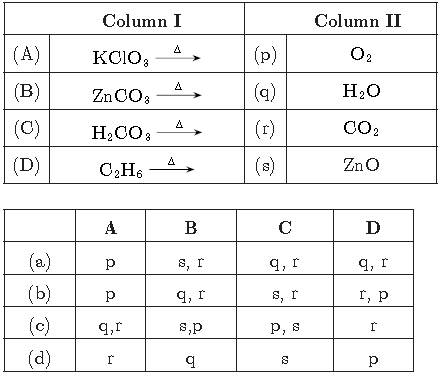
Answer
(a) A-p, B-s, r, C-q, r, D-q, r
Assertion And Reason
Question. Assertion : Stannous chloride is a powerful oxidising agent which oxidises mercuric chloride to mercury.
Reason : Stannous chloride gives grey precipitate with mercuric chloride, but stannic chloride does not do so.
Answer
C
Question. Assertion : Fe2O3 + 2Al → Al2O3 + 2Fe The above chemical equation is an example of displacement reaction.
Reason : Aluminium being more reactive than iron, displaces Fe from its oxide.
Answer
A
Question. Assertion : In the following chemical equation, CuO(s) + Zn(s) → ZnO(s) + Cu(s) Zinc is getting oxidised and copper oxide is getting reduced.
Reason : The process in which oxygen is added to asubstance is called oxidation whereas the process in which oxygen is removed from a substance is called reduction.
Answer
A
Question. Assertion : Quicklime reacts vigorously with water releasing a large amount of heat.
Reason : The above chemical reaction is an exothermic reaction.
Answer
A
Question. Assertion : Photosynthesis is considered as an endothermic reaction.
Reason : Energy gets released in the process of photosynthesis.
Answer
C