Please refer to Assignments Class 12 Chemistry The d – and f – Block Elements Chapter 8 with solved questions and answers. We have provided Class 12 Chemistry Assignments for all chapters on our website. These problems and solutions for Chapter 8 The d – and f – Block Elements Class 12 Chemistry have been prepared as per the latest syllabus and books issued for the current academic year. Learn these solved important questions to get more marks in your class tests and examinations.
The d – and f – Block Elements Assignments Class 12 Chemistry
Question. Which of the following is the reason of Zinc for not exhibiting variable oxidation states ?
(A) Inert pair effect
(B) Completely filled 3d subshell
(C) Completely filled 4s subshell
(D) Common ion effect
Answer
B
Question. The oxidation state of Ni in [Ni(CO)4] is:
(A) 0
(B) 2
(C) 3
(D) 4
Answer
A
Question. Electronic configuration of a transition element X in +3 oxidation state is [Ar]3d5. What is its atomic number?
(A) 25
(B) 26
(C) 27
(D) 24
Answer
B
Question. Which of the following is a diamagnetic ion ?
(Atomic numbers of Sc, V, Mn and Cu are 21, 23, 25 and 29 respectively)
(A) V2+
(B) Sc3+
(C) Cu2+
(D) Mn3+
Answer
B
Question. Lanthanoid contraction is caused due to:
(A) Atomic number
(B) Size of 4f orbitals
(C) Effective nuclear charge
(D) Poor shielding effect of 4f electrons
Answer
D
Question. Which set of ions exhibit specific colours?
(Atomic number of Sc = 21, Ti = 22, V=23, Mn = 25,
Fe = 26, Ni = 28 Cu = 29 and Zn =30)
(A) Sc3+, Ti4+, Mn3+
(B) Sc3+, Zn2+, Ni2+
(C) V 3+, V2+, Fe3+
(D) Ti3+, Ti4+, Ni2+
Answer
C
Question. There are 14 elements in lanthanoid series. Which of the following element belong to this series?
(A) Ce
(B) Sm
(C) Tm
(D) All of the above
Answer
D
Question. In which of the following elements, 5f orbitals are progressively filled?
(a) Alkaline earth metals
(b) Actinoids
(c) Lanthanoids
(d) Transition elements
Answer
B
Question. Gadolinium belongs to 4f series. Its atomic number is 64. Which of the following is the correct electronic configuration of Gadolinium?
(A) [Xe] 4f 75d16s2
(B) [Xe] 4f 65d26s2
(C) [Xe] 4f 86d2
(D) [Xe] 4f95s1
Answer
A
Question. Interstitial compounds are formed when small atoms are trapped inside the crystal lattice of metals. Which of the following is not the characteristic property of interstitial compounds?
(A) They have high melting points in comparison to pure metals.
(B) They are very hard.
(C) They retain metallic conductivity.
(D) They are chemically very reactive.
Answer
D
Question. The electronic configuration of Cu(II) is 3d9 whereas that of Cu(I) is 3d10. Which of the following is correct?
(A) Cu(II) is more stable.
(B) Cu(II) is less stable.
(C) Cu(I) and Cu(II) are equally stable.
(D) Stability of Cu(I) and Cu(II) depends on nature of copper salts.
Answer
A
Question. Which of the following statements is not correct?
(A) La is actually transition element.
(B) In Lanthanide series, ionic radii decrease from La3+ to Lu3+.
(C) La(OH)3 is less basic than Lu(OH)3.
(D) Ionic radii of Zr and Hf are almost similar due to lanthanoid contraction.
Answer
C
Question. Which of the following oxidation state is common for all lanthanoids?
(A) +2
(B) +3
(C) +4
(D) +5
Answer
B
Assertion and Reason Based MCQs
In the following questions, a statement of Assertion (A) followed by a statement of Reason (R) is given. Choose the correct option out of the following choices.
(a) Assertion and Reason both are true, Reason is the correct explanation of Assertion.
(b) Assertion and Reason both are true but Reason is not the correct explanation of Assertion.
(c) Assertion is true, Reason is false.
(d) Assertion is false, Reason is true.
Question. Assertion(A) : [Co(NH3)Br]SO4 gives white precipitate with barium chloride.
Reason(R) : The complex dissociates in the solution to give Br – and SO4 2- .
Answer
C
Question. Assertion (A): (Fe(CN)6]3- ion shows magnetic moment corresponding to two unpaired electrons.
Reason (R): Because it has d2sp3 type hybridisation.
Answer
D
Question. Assertion(A) : [FeF6] 3- is paramagnetic.
Reason (R): F- is a weak field ligand, hence does not cause pairing of electrons.
Answer
A
Question. Assertion (A): Toxic metal ions are removed by the chelating ligands.
Reason (R): Chelate complexes tend to be more stable.
Answer
A
Question. Assertion(A) : According to crystal field theory during complex formation, the d – orbitals split and form two sets of orbitals t2g and eg.
Reason(R) : Splitting of d – orbitals occurs only in case of strong field ligands.
Answer
C
Question. Assertion (A): [Cr(H2O)6]Cl2 and [Fe(H2O)6]Cl2 are reducing in nature.
Reason (R): Unpaired electrons are present in their d-orbitals.
Answer
B
Question. Assertion(A) : Low spin tetrahedral complexes are not formed.
Reason(R) : For tetrahedral complexes, CFSE is lower than pairing energy.
Answer
A
Question. Assertion(A) : [Fe(H2O)6] 2+ is sp3 d 2 hybridised and paramagnetic complex ion.
Reason(R) : It has four unpaired electrons.
Answer
A
Directions: In the following questions, a statement of Assertion (A) is followed by a statement of Reason (R).
Mark the correct choice as:
(A) Both (A) and (R) are true, and (R) is the correct explanation of (A).
(B) Both (A) and (R) are true, but (R) is not the correct explanation of (A).
(C) (A) is true, but (R) is false.
(D) (A) is false, but (R) is true.
Question. Assertion (A): Transition metals have low melting points.
Reason (R): The involvement of greater number of (n – 1)d and ns electrons in the interatomic metallic bonding.
Answer
D
Question. Assertion (A): The highest oxidation state of osmium is +8.
Reason (R): Osmium is a 5d-block element.
Answer
B
Question. Assertion (A): Lanthanoids have poor tendency to form complexes.
Reason (R): Lanthanoids has low density.
Answer
A
Question. Assertion (A): Cu2+ iodide is not known.
Reason (R): Cu2+ oxidises I– to iodine.
Answer
A
Question. Assertion (A): Cu cannot liberate hydrogen from acids.
Reason (R): Cu has positive electrode potential.
Answer
A
Question. Assertion (A): Chromium is an actinoid.
Reason (R): In chromium, 3d orbitals are filled.
Answer
D
Question. Assertion (A): Separation of Zr and Hf is difficult.
Reason (R): Zr and Hf lie in the same group of the periodic table.
Answer
B
Question. Assertion (A): Cerium (Ce) exhibits +4 oxidation state.
Reason (R): Ce4+ has 4f4 electronic configuration which is less stable.
Answer
C
Case-based MCQs
I. Read the passage given below and answer the following questions:
Within the 3d series, manganese exhibits oxidation states in aqueous solution from +2 to +7,ranging from Mn2+(aq) to MnO4 – (aq). Likewise,iron forms both Fe2+(aq) and Fe3+(aq) as wellas the FeO42−4 ion. Cr and Mn form oxoanions CrO2−4, MnO4 −, owing to their willingness to form multiple bonds. The pattern with the early transition metals—in the 3d series up to Mn, and for the 4d, 5d metals upto Ru and Os—is that the maximum oxidation state corresponds to the number of ‘‘outer shell’’ electrons. The highest oxidation states of the 3d metals may depend upon complex formation (e.g., the stabilisation of Co3+ by ammonia) or upon the pH (thus MnO4 2− (aq) is prone to disproportionation in acidic solution). Within the 3d series, there is considerable variation in relative stability of oxidation states, sometimes on moving from one metal to a neighbour; thus, for iron, Fe3+ is more stable than Fe2+, especially in alkaline conditions, while the reverse is true for cobalt. The ability of transition metals to exhibit a wide range of oxidation states is marked with metals such as vanadium, where the standard potentials can be rather small, making a switch between states relatively easy.
In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage.
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(C) Assertion is correct statement but reason is wrong statement.
(D) Assertion is wrong statement but reason is correct statement.
Question. Assertion (A): Fe3+ is more stable than Fe2+.
Reason (R): Fe3+ has 3d5 configuration while Fe2+ has 3d6 configuration.
Answer
A
Question. Assertion (A): The highest oxidation states of the 3d metals depends only on electronic configuration of the metal.
Reason (R): The number of electrons in the (n-1) d and ns subshells determine the oxidation states exhibited by the metal.
Answer
D
Question. Assertion (A): Vanadium had the ability to exhibit a wide range of oxidation states.
Reason (R): The standard potentials of vanadium are rather small, making a switch between oxidation states relatively easy.
Answer
A
Question. Assertion (A): Highest oxidation state is exhibited by transition metals lying in the middle of the series.
Reason (R): The highest oxidation state exhibited corresponds to number of (n−1)d electrons.
Answer
C
Question. Assertion (A): Transition metals like Fe, Cr and Mn form oxoanions.
Reason (R): Oxygen is highly electronegative and has a tendency to form multiple bonds.
Answer
B
II. Read the passage given below and answer the following questions:
The transition metals when exposed to oxygen at low and intermediate temperatures form thin, protective oxide films of upto some thousands of Angstroms in thickness. Transition metal oxides lie between the extremes of ionic and covalent binary compounds formed by elements from the left or right side of the periodic table. They range from metallic to semiconducting and deviate by both large and small degrees from stoichiometry. Since d-electron bonding levels are involved, the cations-exist in various valence states and hence give rise to a large number of oxides. The crystal structures are often classified by considering a cubic or hexagonal close-packed lattice of one set of ions with the other set of ions filling the octahedral or tetrahedral interstices. The actual oxide structures, however, generally show departures from such regular arrays due in part to distortions caused by packing of ions of different size and to ligand field effects. These distortions depend not only on the number of d-electrons but also on the valence and the position of the transition metal in a period or group.
In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage.
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(C) Assertion is correct statement but reason is wrong statement.
(D) Assertion is wrong statement but reason is correct statement.
Question. Assertion (A): CrO crystallises in a hexagonal closepacked array of oxide ions with two out of every three octahedral holes occupied by chromium ions.
Reason (R): Transition metal oxide may be hexagonal close-packed lattice of oxide ions with metal ions filling the octahedral voids.
Answer
D
Question. Assertion (A): Crystal structure of oxides of transition metals often show defects.
Reason (R): Ligand field effect cause distortions in crystal structures.
Answer
A
Question. Assertion (A): Cations of transition elements occur in various valence states.
Reason (R): Large number of oxides of transition elements are possible.
Answer
B
Question. Assertion (A): Transition metals form protective oxide films.
Reason (R): Oxides of transition metals are always stoichiometric.
Answer
C
III. Read the passage given below and answer the following questions:
The d block elements are the 40 elements contained in the four rows of ten columns (3-12) in the periodic table. As all the d block elements are metallic, the term d-block metals is synonymous. This set of d-block elements is also often identified as the transition metals, but sometimes the group 12 elements (zinc, cadmium, mercury) are excluded from the transition metals as the transition elements are defined as those with partly filled d or f shells in their compounds. Inclusion of the elements zinc, cadmium and mercury is necessary as some properties of the group 12 elements are appropriate logically to include with a discussion of transition metal chemistry. The term transition element or transition metal appeared to derive from early studies of periodicity such as the Mendeleev periodic table of the elements. His horizontal table of the elements was an attempt to group the elements together so that the chemistry of elements might be explained and predicted. In this table there are eight groups labeled I-VIII with each subdivided into A and B subgroups. Mendeleev recognized that certain properties of elements in Group VIII are related to those of some of the elements in Group VII and those at the start of the next row Group I. In that sense, these elements might be described as possessing properties transitional from one row of the table to the next.
In the following questions, a statement of assertion followed by a statement of reason is given. Choose the correct answer out of the following choices on the basis of the above passage.
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(C) Assertion is correct statement but reason is wrong statement.
(D) Assertion is wrong statement but reason is correct statement.
Question. Assertion (A): Group 12 elements are not considered as transition metals.
Reason (R): Transition metals are those which have incompletely filled d sub-shell in their compounds.
Answer
A
Question. Assertion (A): All d-block elements are metallic in nature.
Reason (R): The d-block elements belong to Group 3 -12 of the periodic table.
Answer
B
Question. Assertion (A): Group VII elements of Mendeleev periodic table are transition elements.
Reason (R): Group I –VIII in Mendeleev periodic table is divided into two subgroups, A and B.
Answer
D
Question. Assertion (A): Nickel is a transition element that belongs to group 10 and period 4 of the modern periodic table.
Reason (R): Electronic configuration of Nickel is [Ar]3d84s2.
Answer
A
IV. Read the passage given below and answer the following questions:
In transition elements, generally, ions of the same charge in a given series show progressive decrease in radius with increasing atomic number. This is because the new electron enters a d orbital each time the nuclear charge increases by unity. But the radii of the third (5d) series are virtually the same as those of the corresponding members of the second series. This phenomenon is associated with the intervention of the 4f orbitals which must be filled before the 5d series of elements begin. The filling of 4f before 5d orbital results in a regular decrease in atomic radii called Lanthanoid contraction. In these questions, a statement of assertion followed by a statement of reason is given.
Choose the correct answer out of the following choices.
(A) Assertion and reason both are correct statements and reason is correct explanation for assertion.
(B) Assertion and reason both are correct statements but reason is not correct explanation for assertion.
(C) Assertion is correct statement but reason is wrong statement.
(D) Assertion is wrong statement but reason is correct statement.
Following are the transition metal ions of 3d series:
Ti4+, V2+, Mn3+, Cr3+
(Atomic number: Ti = 22, V = 23, Mn = 25, Cr = 24)
Question. Assertion (A): There is irregular trend in ionization energy in the group of lanthanoid series.
Reason (R): Ionization energy in lanthanoid series vary due to lanthanoid contraction.
Answer
A
Question. Assertion (A): Among the given ions, Mn3+ is the most strong oxidising agent.
Reason (R): Mn3+ has an unstable configuration.
Answer
C
Question. Assertion (A): Among the given ions, Cr3+ is the most stable in an aqueous environment.
Reason (R): Cr3+ has half filled t2g3.
Answer
A
Question. Assertion (A): Ti4+ ion is colourless.
Reason (R): All valence electrons are unpaired in Ti4+ ion.
Answer
C
Short Answer Type Questions-I
Question. Which metal in the first transition series exhibits +1 oxidation state most frequently and why ?
Answer.Cu has the electronic configuration 3d10 4s1 .It can easily lose 4s1 electron to give the stable 3d10 configuration .
Question. Why do transition elements exhibit higher enthalpy of atomization ?
Answer.Due to stronger interatomic interaction .
Question. Cu+ is not stable in aqueous solution .Why ?
Answer. Many Cu(I) compounds are unstable in aqueous solution and undergo disproportionation.
2Cu+ → Cu2+ + Cu
The stability of Cu2+ rather than Cu+ is due to more –ve hydration enthalpy of Cu2+ than Cu+ which is much more and compensate for the II ionisation enthalpy .
Question. Co2+is stable in aqueous solution but in the presence of complexing agent ,it is easily
oxidised .Why ?
Answer.In the presence of complexing agent ,oxidation state of Co changes from +2 to +3 due to CFSE which is more and compensate the ΔiH3 .
Question. Why Zn ,Cd and Hg are soft and have low melting and boiling points ?
Answer. Due to weak metallic bonds present in them as all the electrons in d-subshell are paired .
Question. Most of the transition elements are paramagnetic .Why ?
Answer. Due to presence of unpaired electron in (n-1) d subshell.
Question. Name a transition element which does not exhibit variable oxidation states .
Answer. Scandium .
Question. Which of the 3d series of the transition metals exhibits the largest number of oxidation states and why ?
Answer. In 3d series Mn shows the highest oxidation state of +7 as it has maximum no. of unpaired e- .
Question. What are transition elements ?
Answer.Incompletely filled d –orbital in ground state or in any one of their oxidation state.
Question. Why is the highest oxidation state of a metal exhibited in its oxide or fluoride only ?
Answer. Oxygen and fluorine have small size and high electronegativity .Hence, they can oxidize the metal to the highest oxidation state .
Question. Transition elements form colored compounds . Why ?
Answer. Due to d-d transition .
Question. Zr and Hf have almost identical radii?
Answer. Due to filling of 4f orbitals which have poor shielding effect / lanthanoid contraction.
Question. La(OH)3 is a stronger base than Lu(OH)3?
Answer. As the size of lanthanoid elements decreases from La3+ to Lu3+ covalent character of hydroxide increases hence basic strength decreases .
Question. Transition elements form alloys .Why ?
Answer. Due to similar metallic radii .
Question. Why do transition elements shows variable oxidation states ?
Answer.Due to participation of (n-1)d and ns electron in bond formation .
Question. Which is the stronger reducing agent Cr2+ or Fe2+ and why?
Answer. Cr2+ is the stronger reducing agent because in case of Cr2+ to Cr3+ change in configuration is from d4 to d3 and in Fe2+ to Fe3+ the change is from d6 to d5. In medium like water d3 is more stable as compared to d5 due to half filled t2g configuration.
Question. The d1 configuration is very unstable in ions. Why ?
Answer. The ions with d1 configuration have the tendency to lose the only electron present in d-subshell to acquire stable d0 configuration .
Question. Why do transition metals and their compounds show catalytic activity ?
Answer. Because of multiple oxidation state / ability to form complex / having large surface area .
Question. Why Zn ,Cd and Hg are not regarded as transition elements ?
Answer. Do not have partly fiiled d –orbital in ground state or in any one of their oxidation state.
Question. Why transition metals form large number of interstitial compounds ?
Answer. Because small non metallic atoms (H,B,C,N etc) are able to fit in the interstitial sites of transition metal lattice to form interstitial compounds .
Question. Why is Cu2+ ion colored while Zn2+ ion is colorless in aqueous solution ?
Answer.Presence of unpaired e- showing d-d transition in Cu2+ while in Zn2+ there is no unpaired electron .
Question. E0 for Mn3+/Mn2+ is more positive than for Fe3+/Fe2+ . Why ?
Answer. Mn3+has the configuration 3d4 while that of AMn2+ is 3d5 .So Mn3+ easily undergo reduction to Mn2+ having stable 3d5 configuration resulting in higher value of standard reduction potential .
Fe3+ is more stable than Fe2+ because of having 3d5 configuration and reduction to Fe2+ will not be easy resulting in the decreased value of E0 .
Question. Transition elements have high melting and boiling points ?
Answer. Due to strond metallic bond .
Question. The 4d and 5d series of transition metals have more frequent metal –metal bonding in their compounds than 3d series .Explain .
Answer.In the same group of d-block elements ,the 4d and 5d transition elements have larger size than that of 3d element .Thus the valence electrons are less tightly held and hence can form metal metal bond more frequently .
Question. What is meant by ‘disproportionation’ ? Give an example of a disproportionation reaction in aqueous solution.
OR
Suggest reasons for the following features of transition metal chemistry:
(i) The transition metals and their compounds are usually paramagnetic.
(ii) The transition metals exhibit variable oxidation states.
Answer. Disproportionation is the reaction in which an element undergoes self-oxidation and selfreduction simultaneously.
For example –
2Cu+ (aq) → Cu2+ (aq) + Cu(s)
(Or any other correct equation)
Question. What are the transition elements ? Write two characteristics of the transition elements.
Answer. These atoms whose d-orbitals are incomplete in ground state or in one of the most common oxidation state are called transition elements or d-block elements. The valence shell electronic configuration of transition elements is (n-1)d1-10ns1-2 .
Two characteristics of transition elements:
(i) Transition metals show variable oxidation states.
(ii) All transition metals act as catalyst.
Question. (i) Write the formula of an oxo-anion of Manganese (Mn) in which it shows the oxidation state equal to its group number.
(ii) Write the formula of an oxo-anion of Chromium (Cr) in which it shows the oxidation state equal to its group number.
Answer. (i) MnO4–/KMnO4
(ii) Cr2O7 2–/CrO4 2–/K2Cr2O7/K2CrO4
Question. In the following ions:
Mn3+, V3+, Cr3+, Ti4+
(Atomic no: Mn = 25, V = 23, Cr = 24, Ti = 22)
(a) Which ion is most stable in an aqueous solution?
(b) Which ion is the strongest oxidizing agent?
(c) Which ion is colourless?
(d) Which ion has the highest number of unpaired electrons?
Answer. (a) Cr3+
(b) Mn3+
(c) Ti4+
(d) Mn3+
Question. Identify the following:
(i) Transition metal of 3d series that exhibits the maximum number of oxidation states.
(ii) An alloy consisting of approximately 95% lanthanoid metal used to produce bullet, shell and lighter flint.
Answer. (i) Mn
(ii) Mischmetal
Question. Explain the following observations:
(i) Copper atom has completely filled d orbitals (3d10) in its ground state, yet it is regarded as a transition element.
(ii) Cr2+ is a stronger reducing agent than Fe2+ in aqueous solution.
Answer. (i) Because it has incompletely filled d orbitals in one of its oxidation state (Cu2+).
(ii) Cr2+(d4) changes to Cr3+(d3) while Fe2+(d6) changes to Fe3+ (d5). In aqueous medium d3 is more stable than d5.
Question. Identify the following:
(i) Oxo anion of chromium which is stable in acidic medium.
(ii) The lanthanoid element that exhibits +4 oxidation state.
Answer. (i) Cr2O72-
(ii) Cerium
Question. Write one similarity and one difference between the chemistry of lanthanoids and actinoids.
Answer. Similarity: (i) Both show contraction in size.
(ii) Both show irregularity in their electronic configuration. (iii) Both are stable in +3 oxidation state.
Difference: (i) Actinoids are mainly radioactive but lanthanoids are not. (ii) Actinoids show wide range of oxidation states but lanthanoids do not.
(iii) Actinoid contraction is greater than lanthanoid contraction.
Question. Explain the following observation
(i) Zn2+ salt are colourless.
(ii) Copper has exceptionally positive EoM2+/M value.
Answer.
(i) Zinc has no unpaired electrons in its d orbital and has a stable fully filled d orbital. Thus, due to absence of unpaired electrons, there will not be the excitation of electrons from lower energy level to higher level to exhibit complementary colour and hence Zn2+ salts are colourless.
(ii) As copper has high energy of atomisation ΔaH° and low hydration energy ΔhydH°, due to which E° value is positive.
Question. (i) Why Lanthanoids cannot be easily separated?
(ii) Why does Zr (Z=40) and Hf (Z=72) exhibit almost identical atomic radii?
Answer. (i) Due to lanthanoid contraction, the chnge in the atomic or ionic radii of these elements is very small.
So, their chemical properties are similar. Hence,Lanthanoids cannot be easily separated.
(ii) Lanthanoid contraction is responsible for almost same atomic radii of 4d and 5d transition series elements.
Question. Explain the following observation:
(i) Silver atom has completely filled d-orbitals (4d10) in its ground state, yet it is regarded as a transition element.
(ii) E° value for Mn3+/Mn2+ couple is much more positive than Cr3+/Cr2+.
Answer. (i) Silver can exhibit +2 oxidation state, wherein it will have incompletely filled d-orbital.
(ii) Much higher third ionisation energy of Mn where the required change is from d5 to d4.
Question. Name the following:
(i) A transition metal which does not exhibit variation in oxidation state in its compounds.
(ii) A compound where the transition metal is in the +7 oxidation state.
(iii) A member of the lanthanoid series which is well known to exhibit +4 oxidation state.
(iv) Ore used in the preparation of Potassium dichromate.
Answer. (i) Scandium (Sc).
(ii) KMnO4 or any other suitable example.
(iii) Cerium (Ce) or any other example.
(iv) Chromite ore.
Question. Give reasons:
(i) Zn is not regarded as a transition element.
(ii) Cr2+ is a strong reducing agent.
Answer. (i) In both, Zn and Zn2+ ions absence of incompletely filled d-orbital therefore, Zn is not regarded as a transition element.
(ii) Cr2+ has d4 configuration while Cr3+ has more stable d3 (t23 g ) configuration. Thus, Cr has a tendency to acquire Cr3+ due to greater stability of +3 oxidation state. Therefore, Cr2+ acts as a strong a reducing agent.
Short Answer Type Questions-II
Question. Give reasons for the following:
(a) Transition metals show variable oxidation states.
(b) E° value for (Zn2+ /Zn) is negative while that of (Cu2+ /Cu) is positive.
(c) Higher oxidation state of Mn with fluorine is +4 whereas with oxygen is +7.
Answer.
(a) Because of availability of partially filled orbitals and comparable energies of ns and (n – 1)d orbitals.
(b) E° value for (Zn2+/Zn) is negative due to stable completely filled d10 configuration in Zn2+. The positive value of (Cu2+/Cu) accounts for its ability to liberate H2 from acids due to its high enthalpy of atomization and low hydration energy.
(c) Mn can form multiple bonds with oxygen by using 2p orbital of oxygen and 3d orbital of Mn because of which it shows highest oxidation state of +7
with fluorine, Mn cannot form multiple bonds thus shows an oxidation state of +4.
Question. Account for the following:
(i) CuCl2 is more stable than Cu2Cl2.
(ii) Atomic radii of 4d and 5d series elements are nearly same.
(iii) Hydrochloric acid is not used in permanganate titration.
Answer. (i) In CuCl2, Cu is in +2 oxidation state which is more stable due to high hydration enthalpy as compared to Cu2Cl2 in which Cu is in +1 oxidation state.
(ii) Due to lanthanoid contraction.
(ii) Because HCl is oxidised to chlorine.
Question. (i) (a) Transition metals form alloys. Why?
(b) Chromium is typically hard metal but mercury is liquid. Why?
(ii) Based on the data, arrange Fe2+, Mn2+ and Cr2+ in the increasing order of stability of +2 oxidation state.
E°Cr3+/Cr2+ = – 0.4 V
E°Mn3+/Mn2+ = + 1.5 V
E°Fe3+/Fe2+ = + 0.8 V
Answer. (i) (a) Due to the almost similar atomic sizes of elements, atoms can easily occupy the position in the crystal lattice of the other atom of another element (metal). Thus, formation of alloys is the property of transition metals.
(b) Due to the presence of large number of unpaired electrons, metal-metal interaction is strong whereas mercury does not have unpaired electrons and forms weak metallic bonds.
(ii) Cr2+< Fe2+ < Mn2+
Question. (i) Give reasons for the following:
(a) Compounds of transition elements are generally coloured.
(b) MnO is basic while Mn2O7 is acidic.
(ii) Calculate the magnetic moment of a divalent ion in aqueous medium if its atomic number is 26.
Answer. (i) (a) Due to d-d transition.
(b) Due to higher oxidation state of Mn2O7 / Due to high polarizing power of Mn(VII).
(ii) μ = 4(4 +2) = 4.90 B.M.
Question. Give reasons for the following:
(a) Transition metals have high enthalpies of atomization.
(b) Manganese has lower melting point even though it has a higher number of unpaired electrons for bonding.
(c) Ce4+ is a strong oxidising agent.
Answer.
(a) The transition elements have high enthalpies of atomisation because they have large number of unpaired electrons in their atoms. This results in stronger interatomic interaction and stronger bonding between atoms.
(b) Mn has low melting point because of 3d54s2 configuration, which is highly stable and delocalised, so are not available for bonding, as a result interatomic forces becomes weaker. Hence Mn has low melting point bounded half-filled a orbital electrons with nucleus results in strong interatomic interaction.
(c) The formation of Ce4+ is promoted by its noble gas configuration reverting to the common +3 state. E° value for Ce4+/Ce3+ is + 1.74 V thus readily gains an electron and acts as a strong oxidising agent.
Question. The magnetic moment of few transition metal ions are given below:
Metal ion Magnetic moment (BM)
Sc3+ 0.00
Cr2+ 4.90
Ni2+ 2.84
Ti3+ 1.73
(Atomic no. Sc = 21, Ti = 22, Cr = 24, Ni = 28)
Which of the given metal ions:
(i) has the maximum number of unpaired electrons?
(ii) gives colourless aqueous solution?
(iii) exhibits the most stable +3 oxidation state?
Answer. (i) Cr2+
(ii) Sc3+
(iii) Sc3+
Question. Give reasons:
(i) Mn shows the highest oxidation state of +7 with oxygen but with fluorine it shows the highest oxidation state of +4.
(ii) Transition metals show variable oxidation states.
(iii) Actinoids show irregularities in their electronic configurations.
Answer. (i) Mn can form pp -dp bond with oxygen by using 2p orbital of oxygen and 3d-orbital of Mn because of which it shows highest oxidation state of +7.With fluorine, Mn cannot form pp – dp bond thus shows the highest oxidation state of +4.
(ii) Transition metal show variable oxidation state due to comparable energies of ns and (n – 1)d orbitals and partially filled d orbitals. So, both these orbitals take part in the reactions.
(iii) Due to comparable energies of 5f, 6d and 7s orbitals and the relative stabilities of f 0, f 7 and f14 occupancies of the 5f orbitals.
Question. Give reasons:
(a) E° value for Mn3+/Mn2+ couple is much more positive than that for Fe3+/Fe2+.
(b) Iron has higher enthalpy of atomisation than that of copper.
(c) Sc3+ is colourless in aqueous solution whereas Ti3+ is coloured.
Answer. (a) Because Mn2+ is more stable than Mn3+ due to half-filled d5 configuration whereas Fe2+ becomes unstable after loosing an electron from half-filled orbital.
(b) Due to presence of higher number of unpaired electrons in iron, they have stronger metallic bonding. Hence, the enthalpy of atomisation is more of iron than that of copper.
(c) Sc3+ is colourless as it does not contain unpaired electrons to undergo d-d transition while Ti3+ is coloured as it contains unpaired electrons to undergo d-d transition by absorbing light from visible region and radiate complementary colour
Question. Consider the standard electrode potential values (M2+/M) of the elements of the first transition series.
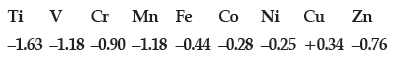
Explain:
(i) E° value for copper is positive.
(ii) E° value of Mn is more negative as expected from the trend.
(iii) Cr3+ is a stronger reducing agent than Fe2+.
Answer. (i) The high energy to transform Cu(s) to Cu2+(aq) is not balanced by its hydration enthalpy.
(ii) Mn2+ has d5 configuration (stable half-filled configuration)
(iii) d5 to d3 occurs in case of Cr2+ to Cr3+. (More stable t2g 3) while it changes from d6 to d5 in case of Fe2+ to Fe3+.
Question. Give reasons for the following:
(i) Transition elements act as catalysts.
(ii) It is difficult to obtain oxidation state greater than two for copper.
(iii) Cr2O72– is a strong oxidising agent in acidic medium whereas WO3 and MoO3 are not.
Answer. (i) Due to large surface area and ability to show variable oxidation states.
(ii) Due to high value of third ionisation enthalpy.
(iii) Mo(VI) and W(VI) are more stable than Cr(VI).
Question. (a) What happens when
(i) Manganate ions (MnO42–) undergoes disproportionation reaction in acidic medium?
(ii) Lanthanum is heated with sulphur?
(b) Explain the following trends in the properties of the members of the First series of transition elements:
(i) E0 (M2+/M) value for copper is positive (+0.34 V) in contrast to the other members of the series.
(ii) Cr2+ is reducing while Mn3+ is oxidising, though both have d4 configuration.
(iii) The oxidising power in the series increases in the order

Answer. (a) (i) MnO42 − ions disproportionate in acidic medium to give permanganate ions and manganese(IV) oxide.
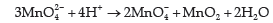
(ii) Lanthanum sulphide if formed.
heat
2La+ 3S → La2 S3
(Deduct overall ½ mark if equation not balanced/ statements not written)
(b) (i) Copper has high enthalpy of atomisation and low enthalpy of hydration, thus the high energy is required to transform Cu(s) to Cu2+(aq) which is not balanced by hydration
enthalpy, therefore Eo(M2+ / M) value for copper is positive (+0.34 V).
(ii) Cr2+ is reducing as its configuration changes from d4 to d3, the latter having more stable half filled t2g level. On the other hand, the change from Mn3+ to Mn2+ results an extra stable d5 configuration.
(iii) This is due to the increasing stability of the species of lower oxidation state to which they are reduced.
Question. Observed and calculated values for the standard electrode potentials of elements from Ti to Zn in the first reactivity series are depicted in figure
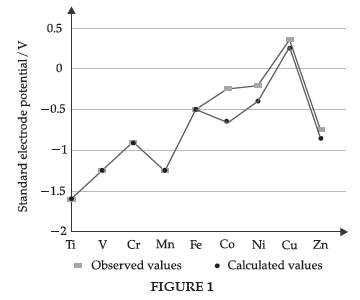
Explain the following observations:
(i) The general trend towards less negative E° values across the series
(ii) The unique behaviour of Copper
(iii) More negative E° values of Mn and Zn
Answer. (i) The general trend towards less negative E° V values across the series is related to the general increase in the sum of the first and second ionisation enthalpies.
(ii) The high energy to transform Cu(s) to Cu2+ (aq) is not balanced by its hydration enthalpy.
(iii) The stability of the half-filled d sub-shell in Mn2+ and the completely filled d10 configuration in Zn2+ are related to their more negative E° V values.
Question. (i) Account for the following:
(a) Cu+ is unstable in an aqueous solution.
(b) Transition metals form complex compounds.
(ii) What is mischmetal? give one use.
Cr2O72- + 8H+ + 3NO2− →
Answer. (i) (a) Because Cu+ undergoes disproportionation as 2Cu+ → Cu + Cu2+.
Hydration enthalpy of Cu2+ is higher than that of Cu+ which compensates the I.E.2 of Cu involved in the formation of Cu2+ ions.
(b) Because of small size of metal, high ionic charge and availability of vacant d-orbital.
(ii) Misch metal is an alloy with composition (95%) lanthanide metal, (5%) ion and traces of S, C, Ca and Al.
It’s alloy of magnesium is used to produce bullets, shells, flints.
Question. Account for the following:
(i) Eu2+ is a strong reducing agent.
(ii) Orange colour of dichromate ion changes to yellow in alkaline medium.
(iii) E°(M2+/M) values for transition metals show irregular variation.
Answer. (i) Eu2+ is a strong reducing agent because Eu3+ is more stable than Eu2+.
(ii) Dichromate ion changes to chromate ion/OH- Cr2O72- (orange) → CrO4 2- (yellow)
(iii) Due to the irregular variation in ionisation enthalpies (sum of [1]st and 2nd ionisation enthalpies), heat of sublimation and enthalpy of hydration/due to irregular electronic configurations from left to right in a period, whichn changes the ionisation potential.
Question. Explain the following:
(a) Out of Sc3+, Co2+ and Cr3+ ions, only Sc3+ is colourless in aqueous solutions. (Atomic no. Co = 27; Sc = 21 and Cr = 24)
(b) The E°Cu2+/Cu for copper metal is positive (+0.34),unlike the remaining members of the first transition series.
(c) La(OH)3 is more basic than Lu(OH)3.
Answer. (a) Co2+ : [Ar]3d7 Sc3+ : [Ar]3d0 Cr3+ : [Ar]3d3
Co2+ and Cr3+ have unpaired electrons. Thus, they are coloured in aqueous solution. Sc3+ has no unpaired electron. Thus it is colourless.
(b) Metal copper has high enthalpy of atomisation and enthalpy of ionisation. Therefore the high energy required to convert Cu(s) to Cu2+(aq) is not balanced by its hydration enthalpy.
(c) Due to lanthanoid contraction the size of lanthanoid ion decreases regularly with increase in atomic size. Thus covalent character between lanthanoid ion and OH– increases from La3+ to Lu3+. Thus the basic character of hydroxides decreases from La(OH)3 to Lu(OH)3.
Question. Give the reasons for following:
(i) Transition elements and their compounds act as catalysts.
(ii) E° value for (Mn+2|Mn) is negative whereas for (Cu+2|Cu) is positive.
(iii) Actinoids show irregularities in their electronic configuration.
Answer.
(i) Transition metals have the ability to adsorb many other substances on their surface and activate them as a result of chemisorption. Transition metals also exhibit a variety of oxidation states, and can change oxidation states relatively easily. This makes transition metals and their compounds good catalysts.
(ii) Eo values are indicative of the stability of the oxidized form of the element. The lower the Eo value, more stable the oxidized form of the element. Mn2+ with a half filled d-subshell (d5) is stable, so Mn is easily oxidized to Mn2+, making the Eo value negative. Cu2+ with a partially filled d subshell (d9) is not stable, and is relatively easily reduced to element form. This makes its Eo value positive.
(iii) The electronic configurations of actinoids show irregularities because the energies of their 5f, 6d,and 7s orbitals are close to each other. Electrons can easily move between these subshells.
Question. Give reasons for the following:
(i) Transition metals form alloys.
(ii) Mn2O3 is basic whereas Mn2O7 is acidic.
(iii) Eu2+ is a strong reducing agent.
Answer.
(i) Transition metals easily form alloys with other transition metals because they have almost similar size. So, they can easily replace each other in the crystal lattice.
(ii) The transition metal oxides in the lower oxidation state of metals are basic in nature and in higher oxidation state they are acidic in nature. The oxidation state of Mn in Mn2O3 is +3 and Mn2O7 has
+7. Therefore, Mn2O3 is basic and Mn2O7 is acidic.
(iii) The common oxidation state of lanthanide metals is +3. Eu2+ is formed by losing the two s electrons acquires half filled (4f7) configuration. But still, they oxidise to their common +3 state. So the Eu2+ loses one electron and is oxidized to Eu3+. So, Eu2+ acts as a strong reducing agent.
Question. Following ions are given:
Cr2+, Cu2+, Cu+, Fe2+, Fe3+, Mn3+
Identify the ion which is
(i) a strong reducing agent.
(ii) unstable in aqueous solution.
(iii) a strong oxidising agent.
Give suitable reason in each.
Answer. (i) Cr2+, because its configuration changes from d4 to d3 and having a half-filled t2g level.
(ii) Cu+ in an aqueous medium energy is required to remove one electron from Cu+ to Cu2+, high hydration energy of Cu2+ compensates for it.
Therefore Cu+ ion in an aqueous solution is unstable.
2Cu+ → Cu2+(aq)+ Cu(s)
(iii) Mn3+, because its configuration changes from Mn3+ to Mn2+ results in the half filled d5 configuration, which has extra stability.
Long Answer Type Questions
Question. (i) (a) How is the variability in oxidation states of transition metals different from that of the p-block elements?
(b) Out of Cu+ and Cu2+, which ion is unstable in aqueous solution and why?
(c) Orange colour of Cr2O72– ion changes to yellow when treated with an alkali. Why?
(ii) Chemistry of actinoids is complicated as compared to lanthanoids. Give two reasons.
Answer. (i) (a) In p-block elements the difference in oxidation state is 2 and in transition metals the difference is 1.
(b) Cu+, due to disproportionation reaction and low hydration enthalpy.
(c) Due to formation of chromate ion/CrO42- ion,which is yellow in colour.
(ii) Actinoids are radioactive, actinoids show wide range of oxidation states.
Question. (a) Give reasons:
(i) Transition metals and their compounds show catalytic activities.
(ii) Separation of a mixture of Lanthanoid elements is difficult.
(iii) Zn, Cd and Hg are soft and have low melting point.
(b) Account for the following:
(i) Ti3+ is coloured whereas Sc3+ is colourless in aqueous solution.
(ii) Cr2+ is a strong reducing agent.
Answer. (a) (i) The catalytic activities of transition metals and their compounds is due to the ability of adopt variable oxidation states and to form complexes. It can also provide a large
surface area for the reactants to be adsorbed.
(ii) Separation of lanthanoid elements is difficult because all lanthanoid elements have almost similar physical as well as chemical properties. Due to the lanthanoid contraction the change in the atomic or ionic radii is very small.
(iii) Zn, Cd and Mg are soft and have low melting point because no d-orbitals are available for metallic bond formation and bonds formed are very weak.
(b) (i) Ti3+ has incomplete d (3d1) orbital whereas Sc3+ has empty (3d°) d-orbital.
(ii) Cr2+ ion can lose electron to form Cr3+, so acts as a strong reducing agent.
Question. (i) Account for the following:
(a) Transition metals show variable oxidation states.
(b) Zn, Cd and Hg are soft metals.
(c) E° value for the Mn3+/Mn2+ couple is highly positive (+1.57 V) as compared to Cr3+/Cr2+.
(ii) Write one similarity and one difference between the chemistry of lanthanoid and actinoid elements.
Answer.(i) (a) The valence electrons of transition metals are in (n-1)d and ns orbitals. As there is almost little energy difference between these orbitals, both the energy levels can be used for bond formation.
Thus, they exhibit variable oxidation states.
(b) Because they contain fully filled d-orbitals, no unpaired d electrons are present resulting in weak metallic bonding.
Question. (i) Complete the following equations:
(a) Cr2O72– + 2OH– →
(b) MnO4– + 4H+ + 3e– →
(ii) Account for the following:
(a) Zn is not considered as a transition element.
(b) Transition metals form a large number of complexes.
(c) The E° value for the Mn3+/Mn2+ couple is much more positive than that for Cr3+/Cr2+ couple.
Answer. (i) (a) Cr2O7 2– + 2OH– → 2 CrO4 2 −+ H2O
(b) MnO4 − + 4H+ + 3e– → MnO2 + 2H2O
(ii) (a) Because Zn/Zn2+ has fully filled d-orbitals.
(b) This is due to smaller ionic sizes, higher ionic charge and availability of d-orbitals
(c) Because Mn2+ is more stable (3d5) than Mn3+(3d4),while Cr+3 is more stable due to t2g 3/d3 configuration.
Question. (i) (a) Which transition element in 3d series has positive E0M2+ /M value and why?
(b) Name a member of lanthanoid series which is well known to exhibit +4 oxidation state and why?
(ii) Account for the following
(a) The highest oxidation state is exhibited in oxoanions of transition metals.
(b) HCl is not used to acidify KMnO4 solution.
(c) Transition metals have high enthalpy of atomisation.
Answer. (ii) (a) Due to high electronegativity and small size, oxygen acts as a strong oxidising agent. This results in oxygen’s ability to oxidise the metal to attain highest oxidation state.
(b) As KMnO4 is a very strong oxidising agent, it oxidizes HCl resulting in evolution of chlorine gas. Therefore, HCl is not used to acidify KMnO4 solution.
Question. The elements of 3d transition series are given as:
Sc Ti V Cr Mn Fe Co Ni Cu Zn
Answer the following:
(i) Write the element which is not regarded as a transition element. Give reason.
(ii) Which element has the highest m.p?
(iii) Write the element which can show an oxidation state of +1.
(iv) Which element is a strong oxidizing agent in +3 oxidation state and why?
Answer. (i) Zn , because it does not have partially filled d-orbital in its ground state or ionic state.
(ii) Cr has the highest melting point. As the number of unpaired electrons increases upto d5 configuration, it results in the increase in the strength of metallic bonds. To break the metallic bond, significant energy is required thus Cr with highest number of unpaired electrons i.e., 6 has the highest melting point.
(iii) Cu can show +1 oxidation state as it can loose one electron present in 4s orbital.
(iv) Mn is a strong oxidising agent in +3 oxidation state because change of Mn3+ to Mn2+ give stable half filled (d5) configuration.
