Please refer to MCQ Questions Chapter 3 Metals and Non-Metals Class 10 Science with answers provided below. These multiple-choice questions have been developed based on the latest NCERT book for class 10 Science issued for the current academic year. We have provided MCQ Questions for Class 10 Science for all chapters on our website. Students should learn the objective based questions for Chapter 3 Metals and Non-Metals in Class 10 Science provided below to get more marks in exams.
Chapter 3 Metals and Non-Metals MCQ Questions
Please refer to the following Chapter 3 Metals and Non-Metals MCQ Questions Class 10 Science with solutions for all important topics in the chapter.
Question. Which of the following metals does not burn on heating?
(a) Magnesium
(b) Copper
(c) Lithium
(d) Sodium
Answer
B
Question. Four metals Al, Zn, Cu and Fe are added in different test tubes containing dilute hydrochloric acid. No bubbles are seen in the case of
(a) Al
(b) Zn
(c) Cu
(d) Fe
Answer
C
Question. Na+ has
(a) 11 protons, 10 electrons
(b) 10 protons, 11 electrons
(c) 12 protons, 11 electrons
(d) 11 protons, 12 electrons
Answer
A
Question. An element reacts with oxygen to give a compound with a high melting point. This compound is also soluble in water. The element is likely to be
(a) calcium
(b) carbon
(c) silicon
(d) iron
Answer
A
Question. Food cans are coated with tin and not with zinc because
(a) zinc is costlier than tin.
(b) zinc has a higher melting point than tin.
(c) zinc is more reactive than tin.
(d) zinc is less reactive than tin.
Answer
C
Question. Which of the following methods is suitable for preventing an iron frying pan from rusting?
(a) Applying grease
(b) Applying paint
(c) Applying a coating of zinc
(d) All of the above
Answer
C
Question. Which of the following property is generally not shown by metals?
(a) Electrical conduction
(b) Sonorous in nature
(c) Dullness
(d) Ductility
Answer
C
Question. The ability of metals to be drawn into thin wire is known as
(a) ductility
(b) malleability
(c) sonorousity
(d) conductivity
Answer
A
Question. Which one of the following metals do not react with cold as well as hot water?
(a) Na
(b) Ca
(c) Mg
(d) Fe
Answer
D
Question. What happens when calcium is treated with water?
(i) It does not react with water.
(ii) It reacts violently with water.
(iii) It reacts less violently with water.
(iv) Bubble of hydrogen gas formed stick to the surface of calcium.
(a) (i) and (iv)
(b) (ii) and (iii)
(c) (i) and (ii)
(d) (iii) and (iv)
Answer
D
Question. Generally metals react with acids to give salt and hydrogen gas. Which of the following acids does not give hydrogen gas on reacting with metals (except Mn and Mg)?
(a) H2SO4
(b) HCl
(c) HNO3
(d) All of these
Answer
C
Question. Generally, metals are solid in nature. Which one of the following metals is found in liquid state at room temperature?
(a) Na
(b) Fe
(c) Cr
(d) Hg
Answer
D
Question. Generally, non-metals are not lustrous. Which of the following non-metal is lustrous?
(a) Sulphur
(b) Oxygen
(c) Nitrogen
(d) Iodine
Answer
D
Question. Which one of the following four metals would be displaced from the solution of its salts by other three metals?
(a) Mg
(b) Ag
(c) Zn
(d) Cu
Answer
B
Question. 2 mL each of concentrated HCl, HNO3 and a mixture of concentrated HCl and concentrated HNO3 in the ratio of 3 : 1 were taken in test tubes labelled as A, B and C. A small piece of metal was put in each test tube. No change occurred in test tubes A and B but the metal got dissolved in test tube C respectively. The metal could be:
(a) Al
(b) Au
(c) Cu
(d) Pt
Answer
B
Question. Although metals form basic oxides, which of the following metals form an amphoteric oxide?
(a) Na
(b) Ca
(c) Al
(d) Cu
Answer
C
Question. Which property of metals is used for making bells and strings of musical instruments like Sitar and Violin?
(a) Sonorous
(b) Malleability
(c) Ductility
(d) Conductivity
Answer
A
Question. Al2O3 + 2NaOH → …… + H2O
(a) Al(OH)3
(b) Na2O
(c) NaAlO2
(d) Al2 NaO2
Answer
C
Question. Which of the following pairs will give displacement reactions?
(a) FeSO4 solution and Copper metal
(b) AgNO3 solution and Copper metal
(c) CuSO4 solution and Silver metal
(d) NaCl solution and Copper metal
Answer
B
Question. Non-metals form covalent chlorides because
(a) they can give electrons to chlorine
(b) they can share electrons with chlorine
(c) they can give electrons to chlorine atoms to form chloride ions
(d) they cannot share electrons with chlorine atoms
Answer
B
Question. Example of an amphoteric oxide is:
(a) Na2O
(b) K2O
(c) Al2O3
(d) MgO
Answer
C
Question. Which one among the following is an acidic oxide?
(a) Na2O
(b) CO
(c) CO2
(d) Al2O3
Answer
C
Question. A student drops pieces of potassium and silver in beakers containing water. The image given below shows the reaction.
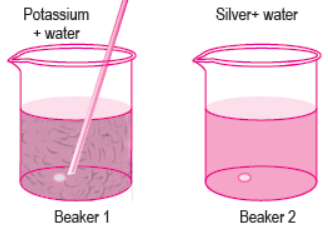
What are the products formed in each beaker?
(a) Beaker 1: K2O and H2O; Beaker 2: No reaction takes place
(b) Beaker 1: KOH and H2O; Beaker 2: Ag2O and H2O
(c) Beaker 1: K2O and H2O; Beaker 2: AgO and H2O
(d) Beaker 1: KOH and H2O; Beaker 2: No reaction takes place
Answer
D
Question. Which product is formed in the chemical reaction between a small strip of magnesium and nitric acid?
(a) MgNO3 and H2O
(b) Mg(NO3)2 and H2O
(c) Mg(NO3)2 and H2
(d) MgNO3 and 2H2
Answer
C
Question. Pick the odd one from the following elements.
(a) Gold
(b) Potassium
(c) Carbon
(d) Platinum
Answer
C
Question. Which of the following statements is false?
(a) Carbon is the most malleable metal.
(b) Copper is a good conductor of electricity.
(c) Aluminium is a good conductor of heat.
(d) Bromine is the only liquid non-metal.
Answer
A
Question. The atomic number of an element ‘X’ is 12. Which inert gas is nearest to X?
(a) He
(b) Ar
(c) Ne
(d) Kr
Answer
C
Question. When zinc reacts with dilute sulphuric acid, a salt is formed with the release of a gas. The gas produced during this puts off a burning candle with a pop sound. The gas evolved during this reaction is:
(a) sulphur dioxide
(b) oxygen
(c) hydrogen
(d) hydrogen sulphide
Answer
C
Question. The property by which metals can be beaten into sheets is known as __________.
(a) ductility
(b) sonority
(c) lusture
(d) malleability
Answer
D
Question. An element X is soft and can be cut with a knife. This is very reactive to air and cannot be kept open in air. It reacts vigorously with water. Identify the element from the following.
(a) Mg
(b) Na
(c) P
(d) Ca
Answer
B
Question. Reaction between X and Y forms compound Z. X loses electron and Y gains electron. Which of the following properties is not shown by Z?
(a) Has high melting point
(b) Has low melting point
(c) Conducts electricity in molten state
(d) Occurs as solid
Answer
B
Question. The electronic configurations of three elements X, Y and Z are X — 2, 8; Y — 2, 8, 7 and Z —
2, 8, 2. Which of the following is correct?
(a) X is a metal
(b) Y is a metal
(c) Z is a non-metal
(d) Y is a non-metal and Z is a metal
Answer
D
Question. The lightest liquid metal is
(a) Hg
(b) Ga
(c) Cs
(d) Fr
Answer
A
Question. The most abundant element in the universe is
(a) Hydrogen
(b) Helium
(c) Carbon
(d) Oxygen
Answer
A
Question. An element ‘X’ is yellow coloured solid, insoluble in water but soluble in carbon disulphide.
It has low melting point 114.5°C. It boils at 445°C and it burns with pale blue flame forming pungent smelling gas ‘Y’ which turns moist blue litmus red and finally colourless. ‘X’ and ‘Y’
are
(a) C, CO2
(b) N, NO2
(c) S, SO2
(d) I2, I2O5
Answer
C
Question. Which of the following oxide(s) of iron would be obtained on prolonged reaction of iron with steam?
(a) FeO
(b) Fe2O3
(c) Fe3O4
(d) Fe2O3 and Fe3O4
Answer
C
Question. A colourless solution kept in a test tube could be
(a) Ferrous sulphate
(b) Copper sulphate
(c) Aluminium sulphate
(d) potassium permanganate
Answer
C
Question. Reddish brown deposits observed on iron nails when these are kept in aqueous solution of copper sulphate is that of
(a) Cu2O
(b) CuO
(c) Cu
(d) CuS
Answer
C
Question. Which of the following metals exist in their native state in nature ?
(i) Cu (ii) Au (iii) Zn (iv) Ag
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (iii) and (iv)
Answer
C
Question. An element ‘X’ reacts with O2 to give a compound with a high melting point. This compound is also soluble in water. The element ‘X’ is likely to be:
(a) iron
(b) calcium
(c) carbon
(d) silicon
Answer
B
Question. An aluminium strip is kept immersed in freshly prepared ferrous sulphate solution taken in a test tube, the change observed is that
(a) Green solution becomes colourless
(b) Lower end of test tube become slightly warm
(c) A colourless gas with the smell of burning sulphur is observed
(d) Light green solution changes to blue.
Answer
A
Question. Aluminium is used for making cooking utensils. Which of the following properties of aluminium are responsible for the same?
(i) Good thermal conductivity (ii) Good electrical conductivity
(iii) Ductility (iv) High melting point
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (i) and (iv)
Answer
D
Question. Which one of the following properties is not generally exhibited by ionic compounds?
(a) Solubility in water
(b) Electrical conductivity in solid state
(c) High melting and boiling points
(d) Electrical conductivity in molten state
Answer
B
Question. Which of the following metals catch fire on reaction with air?
(a) Magnesium
(b) Manganese
(c) Potassium
(d) Calcium
Answer
C
Question. When MgO is dissolved in water, Mg(OH)2 is obtained. The solution thus obtained is ________ in nature.
(a) amphoteric
(b) alkaline
(c) neutral
(d) acidic
Answer
B
Question. Which of the following are not ionic compounds?
(i) KCl (ii) HCl
(iii) CCl4 (iv) NaCl
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (iii) and (iv)
(d) (i) and (iii)
Answer
B
Question. The most abundant metal in the earth’s crust is
(a) Iron
(b) Aluminium
(c) Calcium
(d) Sodium
Answer
B
Question. The poorest conductor of heat among metals is
(a) Lead
(b) Mercury
(c) Calcium
(d) Sodium
Answer
A
Question. Which of the following non-metal is lustrous?
(a) Sulphur
(b) Oxygen
(c) Nitrogen
(d) Iodine
Answer
D
Question. Which of the following metals do not react even with steam?
(a) Silver
(b) Iron
(c) Calcium
(d) Sodium
Answer
A
Question. Generally, non-metals are not conductors of electricity. Which of the following is a good conductor of electricity?
(a) Diamond
(b) Graphite
(c) Sulphur
(d) Fullerene
Answer
B
Question. Electrical wires have a coating of an insulating material. The material, generally used is:
(a) Sulphur
(b) Graphite
(c) PVC
(d) All can be used
Answer
C
Question. Which of the following is correct?
(a) Mg>Al>Zn>Fe
(b) Mg >Al>Fe>Zn
(c) Al>Mg>Zn>Fe
(d) Mg >Zn>Al>Fe
Answer
A
Assertion (A) and Reason (R)
The following questions consist of two statements — Assertion (A) and Reason (R). Answer these questions selecting the appropriate option given below:
(a) Both A and R are true and R is the correct explanation of A.
(b) Both A and R are true but R is not the correct explanation of A.
(c) A is true but R is false.
(d) A is false but R is true.
1. Assertion (A) : Hydrogen gas is not evolved when a metal reacts with nitric acid.
Reason (R) : Nitric acid is a strong oxidising agent.
Answer
A
2. Assertion (A) : Zinc becomes dull in moist air.
Reason (R) : Zinc is coated by a thin film of its basic carbonate in moist air.
Answer
A
3. Assertion (A) : Aluminium is used in making food wrappers.
Reason (R) : Aluminium is highly malleable and has high melting point.
Answer
A
4. Assertion (A) : Zinc oxide is amphoteric in nature.
Reason (R) : Zinc oxide reacts with both acids and bases.
Answer
A
5. Assertion (A) : Magnesium chloride is an ionic compound.
Reason (R) : Metals and non-metals react by mutual transfer of electrons.
Answer
A
6. Assertion (A) : Zinc can easily displace copper on reacting with a solution of copper sulphate.
Reason (R) : Copper is more reactive metal as compared to Zinc.
Answer
C
7. Assertion (A) : MgCl2 is a covalent compound.
Reason (R) : MgCl2 is a good conductor of electricity in molten state.
Answer
D

We hope you liked the above provided MCQ Questions Chapter 3 Metals and Non-Metals Class 10 Science with solutions. If you have any questions please ask us in the comments box below.


