Please refer to MCQ Questions Chapter 10 Haloalkanes and Haloarenes Class 12 Chemistry with answers provided below. These multiple-choice questions have been developed based on the latest NCERT book for class 12 Chemistry issued for the current academic year. We have provided MCQ Questions for Class 12 Chemistry for all chapters on our website. Students should learn the objective based questions for Chapter 10 Haloalkanes and Haloarenes in Class 12 Chemistry provided below to get more marks in exams.
Chapter 10 Haloalkanes and Haloarenes MCQ Questions
Please refer to the following Chapter 10 Haloalkanes and Haloarenes MCQ Questions Class 12 Chemistry with solutions for all important topics in the chapter.
MCQ Questions Answers for Chapter 10 Haloalkanes and Haloarenes Class 12 Chemistry
Question. Which of the following alkyl halide is used as a methylating agent ?
(a) C2H5Br
(b) C6H5Cl
(c) CH3Cl
(d) C2H5Cl
Answer
C
Question. The addition of HI in the presence of peroxide catalyst does not follow anti-Markownikoff addition because
(a) HI is a strong reducing agent
(b) HI bond is too strong to be broken homolytically
(c) I atom combines with H-atom to give back HI
(d) Iodine atom is not reactive enough to add across a double bond
Answer
D
Question. Given,
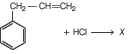
Answer
C
Question. The synthesis of alkyl fluorides is best accomplished by
(a) free radical fluorination
(b) Sandmeyer’s reaction
(c) Finkelstein reaction
(d) Swarts reaction
Answer
B
Question. Which of the following has highest melting point?
(a) Chlorobenzene
(b) o-dichlorobenzene
(c) m-dichlorobenzene
(d) p-dichlorobenzene
Answer
D
Question. Arrange the following compounds in the increasing order of their densities
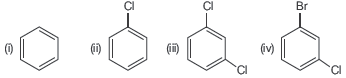
(a) (i) < (ii) < (iii) < (iv)
(b) (i) < (iii) < (iv) < (ii)
(c) (iv) < (iii) < (ii) < (i)
(d) (ii) < (iv) < (iii) < (i)
Answer
A
Question. Consider the following bromides,

The correct order of SN1reactivity is
(a) B > C > A
(b) B > A > C
(c) C > B > A
(d) A > B > C
Answer
A
Question. Which branched chain isomer of the hydrocarbon with molecular mass 72 u gives only one isomer of mono substituted alkyl halide?
(a) Tertiary butyl chloride
(b) Neo -pentane
(c) Iso -hexane
(d) Neo -hexane
Answer
B
Question. The increasing order of reactivity of the following bromides in SN1 reaction is
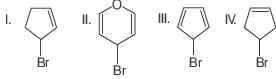
(a) III > I > II > IV
(b) III > II > I > IV
(c) II > III > I > IV
(d) II > I > IV > III
Answer
D
Question. The organic chloro compound which shows complete stereochemical inversion during a SN2 reaction, is
(a) CH3Cl
(b) (C2H5)2CHCl
(c) (CH3)3CCl
(d) (CH3)2CHCl
Answer
A
Question. Preparation of alkyl halides in laboratory is least preferred by
(a) halide exchange
(b) treatment of alcohols
(c) addition of hydrogen halides to alkenes
(d) direct halogenation of alkanes
Answer
D
Question. Match the following and choose the correct option.
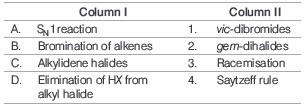
Codes
A B C D
(a) 3 1 2 4
(b) 1 3 2 4
(c) 3 2 1 4
(d) 2 4 3 1
Answer
A
Question. C—Cl bond is stronger than C—I bond because
(a) C—Cl bond is more ionic than C—I
(b) C—Cl bond is polar covalent bond
(c) C—Cl bond is more covalent than C—Cl
(d) C—Cl bond length is longer than C—I
Answer
A
Question. Which of the following compounds will give racemic mixture on nucleophilic substitution by OH− ion?
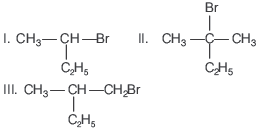
(a) I
(b) I, II, III
(c) II, III
(d) I, III
Answer
A
Question. The increasing order of reactivity of the following halides for the SN1 reaction is
I. CH3CH(Cl)CH2CH3
II. CH3CH2CH2Cl
III. p −H3CO— C6H4 —CH2Cl
(a) (III)<(II)<(I)
(b) (II)< (I)<(III)
(c) (I)<(III)<(II)
(d) (II)<(III)<(I)
Answer
B
Question. Aryl halides are less reactive than alkyl halides towards nucleophile due to
(a) resonance
(b) stability of carbonium ion
(c) high boiling point
(d) None of these
Answer
A
Question. The major product of the following reaction is
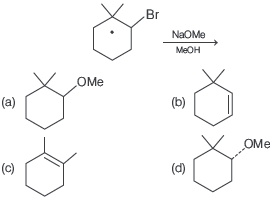
Answer
B
Question. Chlorobenzene can be prepared by reacting aniline with:
(a) hydrochloric acid
(b) cuprous chloride
(c) chlorine in the presence of anhydrous aluminium chloride
(d) nitrous acid followed by heating with cuprous chloride
Answer
D
Question. Which of the following that cannot undergo dehydrohalogenation is
(a) iso-propyl bromide
(b) ethanol
(c) ethyl bromide
(d) None of the above
Answer
B
Question. The final product obtained in the reaction, is
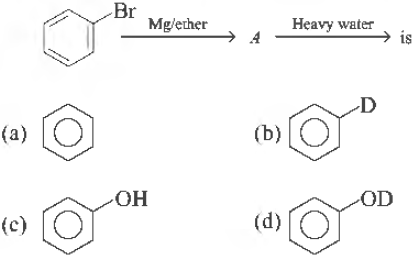
Answer
B
Question. What will be the end product (B) in the following sequence of reactions?
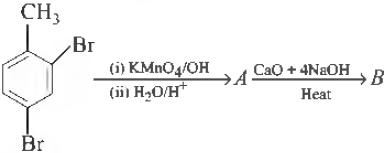
(a) 1, 2-dibromobenzene
(b) 1, 2-dibromobenzaldehyde
(c) 1, 3-dibromobenzene
(d) 1, 4-dibromobenzene
Answer
C
Question. Benzyl chloride (C6H5CH2Cl) can be prepared from toluene by chlorination with
(a) Cl2
(b) SO2Cl2
(c) SOCl2
(d) NaOCl
Answer
A
Question. Compound (A), C8H9Br gives a white precipitate when warmed with alcoholic AgNO3 . Oxidation of (A) gives an acid (B), C8H6O4 . (B) easily forms anyhydride on heating. Identify the compound (A) .
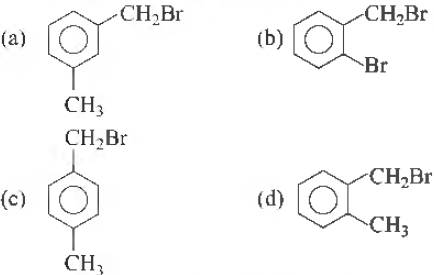
Answer
D
Question. An equimolar mixture of toluene and chlorobenzene is treated with a mixture of cone. H2SO4 and cone. HNO3 . Indicate the correct statement from the following.
(a) p -nitrotoluene is formed in excess
(b) equimolar amounts of p-nitrotoluene and p -nitrochlorobenzene are formed
(c) p-nitrochlorobenzene is formed in excess
(d) m -nitrochlorobenzene is formed in excess
Answer
A
Questionv. Four compounds, toluene (I), o -dichlorobenzene (II), m -dichlorobenzene (III) and p – dichlorobenzene (IV) are arranged in order of increasing dipole moment. The correct order is
(a) IV < I < III < II
(b) I < TI < III < IV
(c) II < IV < III < I
(d) IV < III < II < I
Answer
A
Question. In the chemical reactions, the compounds ‘A’ and ‘B’ respectively are

(a) nitrobenzene and tluorobenzene
(b) phenol and benzene
(c) benzene diazonium chloride and tluorobenzene
(d) nitrobenzene and chlorobenzene
Answer
C
Question. In the preparation of chlorobenzene from aniline, the most suitable reagent is
(a) chlorine in the presence of ultraviolet light
(b) chlorine in the presence of AICl3
(c) nitrous acid followed by heating with Cu2Cl2
(d) HCI and Cu2Cl2
Answer
C
Question. The major product of the following reaction is
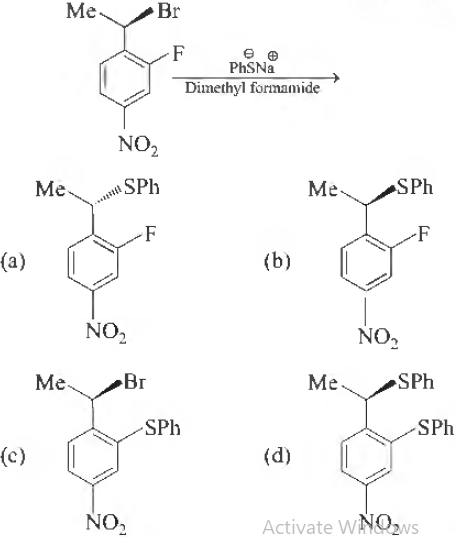
Answer
A
Question. CH3Br + Nu− → CH3 — Nu + Br−
The decreasing order of the rate of the above reaction with nucleophile (Nu− )A to D is
[Nu− = (A) PhO−, (B) AcO−, (C) HO−, (D)CH3O−]
(a) D > C > A > B
(b) D > C > B > A
(c) A > B > C > D
(d) B > D > C > A
Answer
A
Question. How many structures of F are possible?
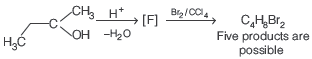
(a) 2
(b) 5
(c) 6
(d) 3
Answer
D
Question. In nucleophilic substitution reaction, order of halogens as incoming (attacking) nucleophile is I− > Br− > Cl−. The order of halogens as departing nucleophile should be
(a) Br− > I− > Cl−
(b) I− > Br− > Cl−
(c) Cl− > Br− > I−
(d) Cl− > I− > Br−
Answer
B
Question. Predict the main product in the given reaction :

(a) Phenyl cyanide
(b) Nitrophenol
(c) Aniline
(d) Hydroxylamine
Answer
C
Question. In alkaline hydrolysis of a tertiary halide by aqueous alkali, if concentration of alkali is doubled, then the reaction rate
(a) will be doubled
(b) will be halved
(c) will remain constant
(d) cannot say anything
Answer
C
Question. Toluene reacts with excess ofCl2 in the presence of sunlight to give a product which on hydrolysis followed by reaction with NaOH gives
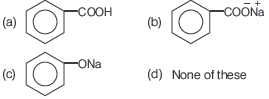
Answer
B
Question. Which halide will be least reactive in respect to hydrolysis ?
(a) Vinyl chloride
(b) Allyl chloride
(c) Ethyl chloride
(d) t-butyl chloride
Answer
A
Question. In S2Nreactions, the correct order of reactivity for the following compounds CH3Cl, CH3CH2Cl, (CH3 )2CHCl and (CH3 )3CCl is
(a) CH3Cl > (CH3)2CHCl > CH3CH2Cl > (CH3)3CCl
(b) CH3Cl > CH3CH2Cl > (CH3)2CH2Cl > (CH3)3CCl
(c) CH3CH2Cl > CH3Cl > (CH3)2CHCl > (CH3) 3CCl
(d) (CH3)2CHCl > CH3CH2Cl > CH3Cl > (CH3)3CCl
Answer
B
Question. Compound A, C8H9Br gives a white precipitate when warmed with alcoholic AgNO3. Oxidation of A gives an acid B, C8H6O4. B easily forms anhydride on heating. Identify the compound A.

Answer
D
Question. Choose the incorrect statement.
(a) An SN1 reaction proceeds with inversion of configuration
(b) An SN2 reaction proceeds with stereochemical inversion
(c) An SN2 reaction follows second order kinetics
(d) The reaction of tert-butyl bromide with OH− follows first order kinetics
Answer
A
Question. A dihaloalkane X, having formula C3H6Cl2, on hydrolysis gives a compound, that can reduce Tollen’s reagent.The compound ‘X’ is
(a) 1, 2- dichloropropane
(b) 1, 1- dichloropropane
(c) 1, 3- dichloropropane
(d) 2, 2- dichloropropane
Answer
B
Question. Which of the following sequence of reagent is best suited for the reaction shown below?

(a) (i) CH3MgBr, H3O; (ii) H+ / D; (iii) HBr/ H O 2 2
(b) (i) CH3MgBr, H3O ; (ii) H+ / D; (iii) HBr
(c) (i) CH3MgBr, H3O ; (ii) HBr;
(d) (i) HBr/R OOR ; (ii) CH3MgBr, H3O+
Answer
A
Question. Choose the incorrect statement.
(a) An SN1 reaction proceeds with inversion of configuration
(b) An SN2 reaction proceeds with stereochemical inversion
(c) An SN2 reaction follows second order kinetics
(d) The reaction of tert-butyl bromide with OH− follows first order kinetics
Answer
A
Question. A solution of (−) -1-chloro -1-phenylethane in toluene racemises slowly in the presence of a small amount of SbCl5, due to the formation of
(a) carbene
(b) carbocation
(c) free radical
(d) carbanion
Answer
B
Question. Which among the following reaction seems to be incorrect?

Answer
A
Question. The chlorine atom in chlorobenzene is ortho and para directing because
(a) resonance effect predominates over inductive effect
(b) inductive effect predominates over resonance effect
(c) both inductive and resonance effects are evenly matched
(d) only resonance effect and not inductive effect is operating
Answer
A
Question. CCl4 is a well known fire extinguisher. However, after using it to extinguish fire, the room should be well ventilated. This is because
(a) it is flammable at higher temperature
(b) it is toxic
(c) it produces phosgene by reaction with water vapour at higher temperature
(d) It is corrosive
Answer
C
Question.

Answer
A
Question. The reaction of toluene withCl2 in the presence of FeCl3 gives predominantly
(a) benzoyl chloride
(b) benzyl chloride
(c) o – and p-chlorotoluene
(d) m-chlorotoluene
Answer
C
Question. The Wurtz-Fittig reaction involves condensation of
(a) two molecules of aryl halides
(b) one molecule of each of aryl halide and alkyl-halide
(c) one molecule of each aryl halide and phenol
(d) two molecules of a alkyl halides
Answer
B
Question. Product on monobromination of this compound is

Answer
B
Question. Iodine from iodoethane can be substituted by cyanide group in a number of ways. Which of the following is true statement?
(a) With AgCN, EtNC while with KCN, EtCN is formed as major product
(b) With AgCN, EtCN and with KCN, EtNC is formed
(c) With either KCN or AgCN, EtNC is formed as major product
(d) With either KCN or AgCN, EtCN is formed as major product
Answer
A
Question. A hydrocarbon has molecular mass = 72. It gives a single monochloride and two dichlorides on further photochlorination. The two dichlorides are
(a) CH3CCl2(CH2)2 CH3 ; CH3(CHCl)2CH2CH3
(b) (CH2)2CCl2CH2CH3 ; (CH3)2(CHCl)2CH3
(c) CHCl2C(CH3)3 ; (CH3)2C(CH2Cl)2
(d) CHCl2CH2CH(CH3)2 CH3CHClCH2CHClCH3
Answer
C
Question. The major product of the following reaction is

Answer
A
Question. The reaction of toluene with Cl2 in the presence of FeCl3 gives X and reaction in presence of light gives Y. Thus, X and Y are
(a) X = benzyl chloride, Y = m- chlorotoluene
(b) X = benzyl chloride, Y = q – chlorotoluene
(c) X = m -chlorotoluene, Y = p- chlorotoluene
(d) X = o and p- chlorotoluene, Y = trichloromethyl benzene
Answer
D
Question. The compound formed on heating chlorobenzene with chloral in the presence of concentrated sulphuric acid is
(a) gammexane
(b) DDT
(c) freon
(d) hexachloroethane
Answer
B
Question. Bottles containing C6H5I and C6H5CH2I lost their original lables. They were labelled A and B for testing. A and B were separately taken in test tubes and boiled with NaOH solution. The end solution in each tube was made acidic with dilute HNO3 and then some AgNO3 solution was added. Substance B gave a yellow precipitate. Which one of the following statements is true for this experiment?
(a) A was C6H5CH2I
(b) A was C6H5CH2I
(c) B was C6H5CH2I
(d) Addition of HNO3 was unnecessary
Answer
A
Question. An organic compound C5H9Br (A) which readily decolourises bromine water and cold alkaline KMnO4 solution and gives C5H11Br (B) on catalytic hydrogenation. The reaction of A with alcoholic KOH first and then with NaNH2 produces C with evolution of NH3. C reacts with Lindlar’s catalyst to give D and on reaction with Na in liquid NH3 produces E. D and E are isomeric. The compound A is
Br
|
(a) CH3CH2CH == C — CH3
Br
|
(b) CH3CH2 — C == CHCH3
(c) Both (a) and (b)
(d) Neither (a) nor (b)
Answer
C
Question. Which is not the correct statement?
(a) Chloretone is an insecticide
(b) COCl2 is called phosgene
(c) Chloropicrin is used as an insecticide
(d) CCl4 is used as fire extinguisher under the name pyrene
Answer
A
Question. (X ) on treatment with sodium hydroxide followed by the addition of silver nitrate give white precipitate at room temperature which is soluble in NH4OH. (X) can be
(a) chlorobenzene
(b) ethyl bromide
(c) benzyl chloride
(d) vinyl chloride
Answer
C
Question. One mole of hydrocarbon (A) reacts with 1 mole of bromine giving a dibromo compound C5H10Br2. (A) on treatment with cold dilute alkaline KMnO4 solution forms a compound C5H12O2. On ozonolysis, (A) gives equimolar quantities of propanone and ethanal. The compound (A) is
(a) (CH3)2C == CHCH3
(b) (CH3)2CH —CH2CH3
(c) CH3CH2CH == CHCH3
(d) CH3 —CH— CH == CH2
|
CH3
Answer
A

We hope you liked the above provided MCQ Questions Chapter 10 Haloalkanes and Haloarenes Class 12 Chemistry with solutions. If you have any questions please ask us in the comments box below.

