Please refer to MCQ Questions Chapter 1 The Solid State Class 12 Chemistry with answers provided below. These multiple-choice questions have been developed based on the latest NCERT book for class 12 Chemistry issued for the current academic year. We have provided MCQ Questions for Class 12 Chemistry for all chapters on our website. Students should learn the objective based questions for Chapter 1 The Solid State in Class 12 Chemistry provided below to get more marks in exams.
Chapter 1 The Solid State MCQ Questions
Please refer to the following Chapter 1 The Solid State MCQ Questions Class 12 Chemistry with solutions for all important topics in the chapter.
MCQ Questions Answers for Chapter 1 The Solid State Class 12 Chemistry
Question. A compound of ‘A’ and ‘B’ crystallises in a cubic lattice in which ‘A · atoms occupy the lattice points at the comers of the cube. The ‘B · atoms occupy the centre of each face
(a) 2
(b) 4
(c) 6
(d) 8
Answer
C
Question. The packing efficiency of the two dimensional square unit cell shown below is

(a) 39.27%
(b) 68.02
(c) 74.05%
(d) 78.54%
Answer
D
Question. Percentage of free space in cubic close packed structure and in body centred packed structure are respectively
(a) 30% and 26%
(b) 26% and 32%
(c) 32% and 48%
(d) 48% and 26%
Answer
B
Question. The 8 : 8 type of packing is present in
(a) MgF2
(b) CsCI
(c) KCl
(d) NaCl
Answer
C
Question. Graphite is a
(a) molecular solid
(b) covalent solid
(c) ionic solid
(d) metallic solid
Answer
B
Question. In a cubic structure of diamond which is made from X and Y, where X atoms are at the comers of the cube and Y at the face centres of the cube. The molecular formula of the compound is
(a) X2Y
(b) X3Y
(c) XY2
(d) XY3
Answer
A
Question. Graphite is a
(a) molecular solid
(b) covalent solid
(c) ionic solid
(d) metallic solid
Answer
B
Question. The ability of a given substance to assume two or more crystalline structure is called
(a) amorphism
(b) isomorphism
(c) polymorphism
(d) isomerism
Answer
C
Question. The number of hexagonal faces that are present in a truncated octahedron is
(a) 2
(b) 4
(c) 6
(d) 8
Answer
D
Question. In a compound, atoms of element Y fom1 ccp lattice and those of element X occupy 213rd of tetrahedral voids. The formula of the compound will be
(a) X4Y3
(b) X2Y3
(c) X2Y
(d) X3Y4
Answer
A
Question. The unit cell with dimensions α = β = ϒ = 90° , a = b ≠ c is
(a) cubic
(b) triclinic
(c) hexagonal
(d) tetragonal
Answer
D
Question. Number of atoms in the unit cell of Na (bee type crystal) and Mg (fee type crystal) are respectively
(a) 4, 4
(b) 4, 2
(c) 2, 4
(d) 1, 1
Answer
C
Question. Coordination number of Zn in ZnS (zinc blende) is
(a) 6
(b) 4
(c) 8
(d) 12
Answer
B
Question. A body centric cubic lattice is made up of two different types of atoms A and B. Atom A occupies the body centre and B occupying the comer positions. One of the comers is left unoccupied per unit cell. Empirical formula of such a solid is
(a) AB
(b) A2B3
(c) A5B7
(d) A8B1
Answer
D
Question. Experimentally it was found that a metal oxide has formula M0.98 O. Metal M, present as M2+ and M3+ in its oxide. Fraction of the metal which exists as M3+ would be
(a) 7.01%
(b) 4.08%
(c) 6.05%
(d) 5.08%
Answer
B
Question. What will be the percentage of free space in bee lattice ?
(a) 32
(b) 68
(c) 72
(d) no free space
Answer
A
Question. A solid has a structure in which, atoms of W,O and Na are located respectively at the comers, centre of edge and at the centre of the cubic lattice. The compound is
(a) NaWO2
(b) NaWO3
(c) Na2WO3
(d) NaWO4
Answer
B
Question. For a crystal system a = b = c and α = β = ϒ = 90°
(a) tetragonal
(b) hexagonal
(c) rhombohedral
(d) monoclinic
Answer
C
Question. In NaCl unit cell, all the ions lying along the axis as shown in the figure are removed.
Then the number of Na+ and Cl– ions remaining in the unit cell are

(a) 4 and 4
(b) 3 and 3
(c) 1 and 1
(d) 4 and 3
Answer
A
Question. Glass isa
(a) micro-crystalline solid
(b) supercooled liquid
(c) gel
(d) polymeric mixture
Answer
B
Question. Iodine is a
(a) electrovalent solid
(b) atomic solid
(c) molecular solid
(d) covalent solid
Answer
C
Question. A compound with cubic structure is made of elements A and B. A atoms are at the comers of the cube and B atoms are at the face centres. The simplest fonnula of compound is
(a) A5B
(b) AB3
(c) AB
(d) AB6
Answer
B
Question. In a face centred cubic lattice, atom A occupies the corner positions and atom B occupies the face centre positions. If one atom of B is missing from one of the face centred points, the formula of the compound is
(a) A2B
(b) AB2
(c) A2B2
(d) A2B5
Answer
D
Question. Which set of characteristics of ZnS crystal is correct?
(a) Coordination number (4 : 4), ccp, Zn2+ ion in the alternate tetrahedral voids
(b) Coordination number (6 : 6), hep, Zn2+ ion in all tetrahedral voids
(c) Coordination number (6 : 4), hep, Zn2+ ion in all octahedral voids
(d) Coordination number (4 : 4), ccp, Zn2+ ion in all tetrahedral voids
Answer
A
Question. Arrangement of sulphide ions in zinc blende is
(a) sc
(b) hep
(c) bee
(d) fee
Answer
D
Question. A compound MpXq has cubic close packing ( ccp) arrangement of X. Its unit cell structure is shown below. The empirical fom1ula of the compound, is

(a) Mt3q5X
(b) MX2
(c) M2X
(d) M5X14
Answer
B
Question. aBarium titanate has the perovskite structure, i.e. a cubic lattice with Ba2+ ions at the comers of the unit cell, oxide ions at the face centres and titanium ions at the body centre. The molecular fonnula of barium titanate is
(a) BaTiO3
(b) BaTiO4
(c) BaTiO2
(d) BaTiO
Answer
A
Question. In a face-centred cubic arrangement of A and B atoms at the face centred, one of A atom is missing from one corner in unit cell. The simplest formula of the compound is
(a) A24B7
(b) A7B24
(c) A7B4
(d) AB2
Answer
B
Question. Which is not the correct statement for ionic solids in which positive and negative ions are held by strong electrostatic attractive forces?
(a) The radius r+ / r– increases as coordination number increases
(b) As the difference in size of ions increases, coordination number increases
(c) When coordination number is eight, the r+ / r– ratio lies between 0.225 to 0.414
(d) In ionic solid of the type AX (ZnS, Wurtzite), the coordination number of Zn2+ and S2- respectively are 4 and 4
Answer
C
Question. A compound is formed by elements A and B. This crystallises in the cubic structure where the A atoms are at the comers of the cube and B atoms are at the body centres. The sinlplest formula of the compound ?
(a) AB
(b) A6B
(c) A8B4
(d) AB6
Answer
B
Question. A solid compound contains X, Y and Z atoms in a cubic lattice with X atom occupying the comers. Y atoms in the body centred positions and Z atoms at the centres of faces of the unit cell. What is the empirical formula of the compound?
(a) XY2Z3
(b) XYZ3
(c) X2Y2Z3
(d) X8YZ6
(e) XYZ
Answer
D
Question. Number of atoms per unit cell of bee is
(a) 1
(b) 2
(c) 8
(d) 4
Answer
B
Question. The lattice points of a crystal of hydrogen iodide are occupi ed by
(a) HI molecules
(b) H atoms and I atoms
(c) H+ cations and r anions
(d) H2 molecules and I2 molecules
Answer
A
Question. Which of the following is not correct for ionic crystals ?
(a) They possess high melting point and boiling point
(b) All are electrolyte
(c) Exhibit the property of isomorphism
(d) Exhibit directional properties of the bond
Answer
D
Question. K.Br crystallises in NaCl type of crystal lattice and its density is 2.75 g/cm3 . Number ofunit cell of KBr are
(a) 3.6 x 1018
(b) 3.6 x 1016
(c) 2.34 x 1023
(d) 6.02 x 1024
Answer
A
Question. In NaCl crystal each CI– ion is surrounded by
(a) 4 Na+ ions
(b) 6 Na+ ions
(c) 1 Na+ ion
(d) 2 Na+ ions
Answer
B
Question. What is the structure of NaCl?
(a) bee
(b) fee
(c) Interpenetrating fee
(d) None of these
Answer
C
Question. Which of the following exists as covalent crystals in the solid state?
(a) Iodine
(b) Silicon
(c) Sulphur
(d) Phosphorus
Answer
B
Question. What is the coordination number of body centred cube?
(a) 8
(b) 6
(c) 4
(d) 12
Answer
A
Question. Which of the following statements is not correct?
(a) The unit of surface tension is dynes cm-1
(b) The unit of viscosity coefficient ofa liquid is ‘poise’
(c) CsCl crystallises in body centred cubic type of lattice
(d) The coordination number of S2- in ZnS is 6
Answer
D
Question. The 8 : 8 type of packing is present in
(a) MgF2
(b) CsCI
(c) KCl
(d) NaCl
Answer
C
Question. In a cubic structure of diamond which is made from X and Y, where X atoms are at the comers of the cube and Y at the face centres of the cube. The molecular formula of the compound is
(a) X2Y
(b) X3Y
(c) XY2
(d) XY3
Answer
A
Question. Which one of the following is a covalent crystal?
(a) Rock salt
(b) rce
(c) Quartz
(d) Dry ice
Answer
C
Question. A metal has bee structure and the edge length of its unit cell is 3.04 A. The volume of the unit cell in cm3 will be
(a) 1.6 x 1021 cm3
(c) 6.02 x 10-23 cm3
(b) 2.81 x 10-23 cm3
(d) 6.6 x 10-24 cm3
Answer
B
Question. The ammgement of X– ions around A+ ion in solid AX is given in the figure (not drawn to scale). If the radius of x is 250 pm, the radius of A+ is
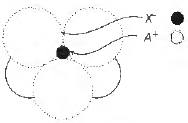
(a) 104 pm
(b) 125 pm
(c) 183 pm
(d) 57 pm
Answer
A
We hope you liked the above provided MCQ Questions Chapter 1 The Solid State Class 12 Chemistry with solutions. If you have any questions please ask us in the comments box below.


