Please refer to Assignments Class 10 Science Assignment on Experiments with solved questions and answers. We have provided Class 10 Science Assignments for all chapters on our website. These problems and solutions for Assignment on Experiments Class 10 Science have been prepared as per the latest syllabus and books issued for the current academic year. Learn these solved important questions to get more marks in your class tests and examinations.
Assignment on Experiments Assignments Class 10 Science
Experiment – 1
Question. A student placed a few drops of a liquid over a portion of the blue litmus paper as shown here. He observed that the blue litmus paper turned red. The liquid could be

(a) dilute hydrochloric acid
(b) dilute sodium hydroxide
(c) dilute sodium bicarbonate solution
(d) water.
Answer
A
Question. A student took two test tubes containing 2 mL of dilute hydrochloric acid and added zinc granules to test tube (A) and solid sodium carbonate to test tube (B) as shown below.
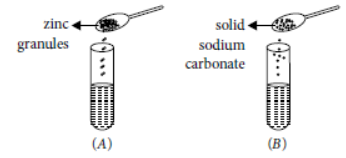
The correct observation would be
(a) rapid reaction in both the test tubes
(b) slow reaction in (A) and rapid reaction in (B)
(c) rapid reaction in (A) but a slow reaction in (B)
(d) no reaction in any of the test tube.
Answer
A
Question. A student was provided with a pH chart by the teacher and asked to observe the colours corresponding to pH 1 and 14 respectively.
The correct answer would be
(a) yellow, green
(b) violet, orange
(c) red, blue
(d) blue, mustard.
Answer
C
Question. Which one of the following setups is the most appropriate for the evolution of hydrogen gas and its identification?
(a) 1
(b) 2
(c) 3
(d) 4
Answer
B
Question. A student adds a few drops of the universal indicator to a solution of dilute hydrochloric acid in the way shown here.
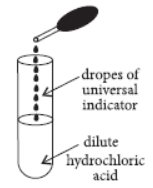
He would observe that the colour of the solution changes from colourless to
(a) red
(b) yellow
(c) violet
(d) green.
Answer
A
Question. To a sample of turmeric adulterated with metanil yellow, concentrated hydrochloric acid was added. The colour of the reaction mixture
(a) disappeared
(b) remained the same
(c) became pink
(d) became black.
Answer
B
Question. On adding a few drops of universal indicator to three unknown colourless solutions (P), (Q) and (R), taken separately in three test tubes shown in the following diagrams, a student observed the changes in colour as green in (P), red in (Q) and violet in (R).
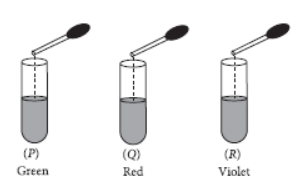
The decreasing order of pH of the solutions taken is
(a) P > Q > R
(b) R > P > Q
(c) Q > P > R
(d) R > Q > P
Answer
B
Question. On putting few drops of an unknown liquid on pH strip, the colour of pH strip changed to green. The liquid taken is likely to be
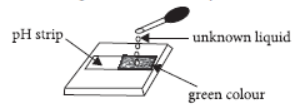
(a) water
(b) dilute hydrochloric acid
(c) lemon juice
(d) dilute sodium hydroxide solution.
Answer
A
Question. When dilute hydrochloric acid is added to granulated zinc placed in a test tube, the observation made is
(a) the surface of the metal turns shining
(b) the reaction mixture turns milky
(c) odour of chlorine is observed
(d) a colourless and odourless gas evolves with bubbles.
Answer
D
Question. Solid sodium bicarbonate was placed on a strip of pH paper. The colour of the strip
(a) turned blue
(b) did not change
(c) turned green and suddenly yellow
(d) turned light pink.
Answer
B
Question. You have four test tubes, A, B, C, and D containing sodium carbonate, sodium chloride, lime water and blue litmus solutions respectively. Out of these the material of which test tube/test tubes would be suitable for the correct test of acetic/ethanoic acid.
(a) Only A
(b) A and B
(c) B and C
(d) A and D
Answer
D
Question. A student adds a few drops of the universal indicator to a dilute solution of sodium bicarbonate taken in a test tube.
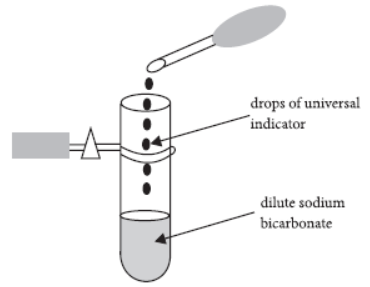
Which of the following colours, would be observe
(a) blue
(b) green
(c) mustard
(d) yellow.
Answer
A
Question. A student added dilute HCl to Zn granules taken in a test tube. The correct observation would be
(a) Zn granules turned green
(b) formation of a precipitate
(c) evolution of gas
(d) no change.
Answer
C
Question. 10 mL of HCl and 10 mL of NaOH solutions are contained in two separate beakers, labelled I and II respectively. On adding zinc granules to both, it is observed that at room temperature
(a) gas is evolved vigorously in both
(b) gas is evolved vigorously in beaker I but not in beaker II
(c) gas is evolved in beaker II but not in beaker I
(d) no gas is evolved in either of the two beakers.
Answer
B
Question. When you prepare 20% sodium hydroxide solution in a beaker containing water, then while preparing the solution you record certain observations. Select from the following the observations which are correct.
I. Sodium hydroxide is in the form of pellets/flakes.
II. It dissolves in water readily.
III. The beaker appears cold when touched from outside immediately after adding sodium hydroxide to water.
IV. When red litmus paper is dipped into the solution, it turns blue.
(a) I, II and III
(b) II, III and IV
(c) III, IV and I
(d) I, II and IV
Answer
D
Question. A colourless liquid sample was tested with pH paper strip. The colour of the strip changed to reddish pink. The sample could be
(a) tap water
(b) sodium hydroxide solution
(c) distilled water
(d) ethanoic acid solution.
Answer
D
Question. A student takes some zinc granules in a test tube and adds dilute hydrochloric acid to it.
He would observe that the colour of the zinc granules changes to
(a) white
(b) black
(c) brown
(d) yellow.
Answer
B
Experiment – 2
Question. When a cleaned iron nail is placed in copper sulphate solution, the colour of solution changes to
(a) blue
(b) red
(c) pale violet
(d) pale green.
Answer
D
Question. A student strongly heats hydrated ferrous sulphate salt in a dry test tube. He would observe a
(a) yellow residue
(b) brown residue
(c) light green residue
(d) white residue.
Answer
B
Question. Iron nails were dipped in an aqueous solution of copper sulphate. AFTer about 30 minutes, it was observed that the colour of the solution changed from
(a) colourless to light green
(b) blue to light green
(c) blue to colourless
(d) green to blue.
Answer
B
Experiment – 3
Question. A student took four test tubes I, II, III and IV containing aluminium sulphate, copper sulphate, ferrous sulphate and zinc sulphate solutions respectively. He placed an iron strip in each of them. He found a brown deposit formed in test tube
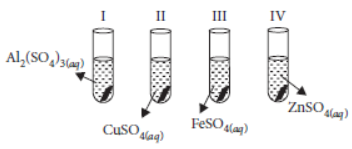
(a) I
(b) II
(c) III
(d) IV
Answer
B
Question. The colours of aqueous solutions of CuSO4 and FeSO4 as observed in the laboratory are
(a) pale green and light blue respectively
(b) dark blue and pale green respectively.
(c) light blue and dark green respectively
(d) dark blue and dark green respectively
Answer
B
Question. To show that zinc is a more active metal than copper, the correct procedure is to
(a) add dilute nitric acid on strips of both the metals
(b) observe transmission of heat through strips of zinc and copper
(c) prepare solution of zinc sulphate and hang strip of copper into it
(d) prepare solution of copper sulphate and hang strip of zinc into it.
Answer
D
Question. Four students A, B, C and D noted the initial colour of the solutions in beaker I, II, III and IV. After inserting zinc rods in each solution and leaving it undisturbed for two hours, noted the colour of each solution again.
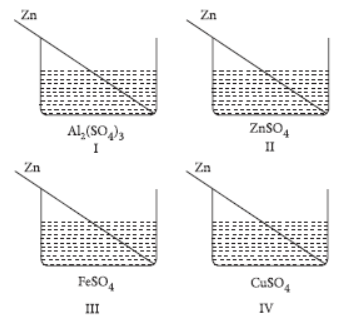
They recorded their observations in the form of table given below :

Which student noted the colour change in all the four beakers correctly?
(a) A
(b) B
(c) C
(d) D
Answer
A
Question. On adding a clean aluminium strip to an aqueous solution of copper sulphate, it is observed that the colour of the solution changes from
(a) blue to colourless
(b) colourless to blue
(c) blue to green
(d) green to blue.
Answer
A
Question. An aqueous solution of zinc sulphate was taken in four test tubes. Zinc, Iron, Copper and Aluminium pieces were dropped into separate test tubes as given below :
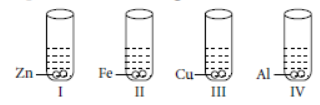
A reaction will be observed in test tubes
(a) I and II
(b) only IV.
(c) I and III
(d) II and III
Answer
B
Question. A student prepared an aqueous solution of CuSO4 in beaker X and an aqueous solution of FeSO4 in beaker Y. He then dropped some iron pieces in beaker X and some zinc pieces in beaker Y. After about 10 hours he observed that the solutions in X and Y respectively appear
(a) blue and green
(b) colourless and pale green
(c) colourless and light blue
(d) greenish and colourless.
Answer
D
Question. The aqueous solutions of copper sulphate and zinc sulphate appear
(a) blue and green respectively
(b) green and colourless respectively
(c) blue and brown respectively
(d) blue and colourless respectively.
Answer
D
Question. Solutions of copper sulphate, iron sulphate and zinc sulphate are prepared and marked I, II and III respectively. Few pieces of aluminium are added to each solution. After some time a change will be observed in
(a) I and II
(b) II and III
(c) III and I
(d) All the three.
Answer
D
Question. A student takes Cu, Al, Fe and Zn strips, separately in four test tubes labelled as I, II, III and IV respectively. He adds 10 mL of freshly prepared ferrous sulphate solution to each test tube and observes the colour of the metal residue in each case.
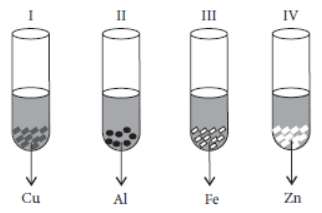
He would observe a black residue in the test tube
(a) (I) and (II)
(b) (I) and (III)
(c) (II) and (III)
(d) (II) and (IV)
Answer
D
Question. A student puts one big iron screw each in four test tubes containing aqueous solutions of aluminium sulphate, copper sulphate, ferrous sulphate and zinc sulphate. After some time he observed a reddish brown coating only on the surface of the screw which was put in the solution of
(a) aluminium sulphate
(b) copper sulphate
(c) ferrous sulphate
(d) zinc sulphate.
Answer
B
Question. A cleaned aluminium foil was placed in an aqueous solution of zinc sulphate. When the aluminium foil was taken out of the zinc sulphate solution after 15 minutes, its surface was found to be coated with a silvery grey deposit. From the above observation it can be concluded that
(a) aluminium is more reactive than zinc
(b) zinc is more reactive than aluminium
(c) zinc and aluminium both are equally reactive
(d) zinc and aluminium both are non-reactive.
Answer
A
Question. Some crystals of ferrous sulphate were dissolved in distilled water. The colour of the solution obtained was
(a) dark green
(b) pale green
(c) light blue
(d) dark blue.
Answer
B
Question. On adding zinc granules to freshly prepared ferrous sulphate solution, a student observes that
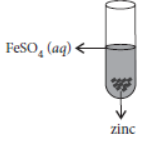
(a) a dull brown coating is formed
(b) a black coating is formed
(c) a grayish coating is formed
(d) no coating is formed.
Answer
C
Question. A student took four test tubes containing solution of different colours marked I, II, III and IV as shown below. The test tubes, containing copper sulphate solution and ferrous sulphate solution, could be the tubes
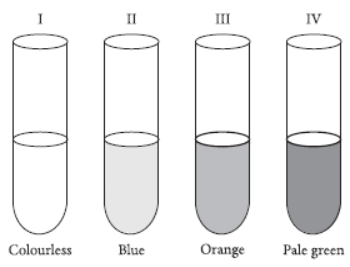
(a) I and III
(b) II and III
(c) III and IV
(d) II and IV.
Answer
D
Question. The colour of an aqueous solution of zinc sulphate as observed in the laboratory is
(a) green
(b) yellow
(c) colourless.
(d) blue
Answer
C
Question. A student took Cu, Al, Fe and Zn strips separately in four test tubes labelled I, II, III and IV. He added 10 mL of freshly prepared ferrous sulphate solution to each test tube as shown below :
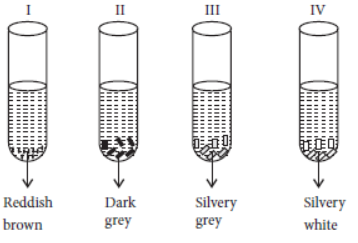
Black residue would be obtained in test tubes
(a) I and II
(b) I and III
(c) II and III
(d) II and IV
Answer
D
Question. When an aluminium strip is kept immersed in freshly prepared ferrous sulphate solution taken in a test tube, the change which is observed is
(a) the green solution slowly turns brown
(b) the lower end of the test tube becomes slightly warm
(c) a colourless gas with smell of burning sulphur is observed
(d) light green solution changes to blue.
Answer
B
Experiment – 4
Question. Three students X, Y and Z, while performing an experiment to study the dependence of current on the potential difference across a resistor, connect the ammeter (A), the battery (B), the key (K) and the resistor (R) in series in the following three different orders.
X → B, K, R, A, B
Y → B, A, K, R, B
Z → B, R, K, A, B
Who has connected them in the correct order?
(a) X
(b) Y
(c) Z
(d) All of them
Answer
D
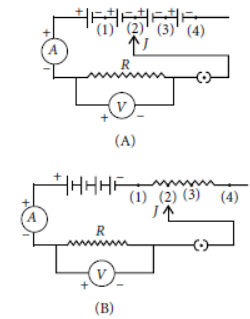
Question. To study the dependence of current (I) on the potential difference (V) across a resistor R, two students used the two set ups shown in figure A and B respectively. They kept the contact point J in four different positions, marked (1), (2), (3) and (4) in the two figures.

For the two students, the ammeter and voltmeters readings will be maximum when the contact J is in the position
(a) (4) in both the set ups
(b) (1) in both the set ups
(c) (4) in set up A and (1) in set up B
(d) (1) in set up A and (4) in set up B
Answer
C
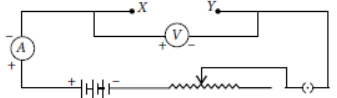
Question. Out of the four given circuits for studying the dependence of the current on the potential difference across a resistor, the circuit that has been correctly drawn is
(a) A
(b) B
(c) C
(d) D
Answer
B
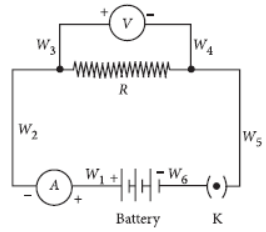
Question. A student sets up the circuit, for studying the dependence of current (I) flowing, on the applied potential difference (V), in the manner shown. The ammeter and the voltmeter, in his circuit, have been checked and found to be correct. On closing the key K, he observes a deflection in the ammeter but no deflection in the voltmeter. This could be due to a loose connection or break in the wire

(a) W1 or W2
(b) W3 or W4
(c) W5 or W6
(d) W6 or W1
Answer
B
Question. The following ‘precautions’ were listed by a student in the experiment on study of ‘dependence of current on potential difference’.
(A) Use copper wires as thin as possible for making connections.
(B) All the connections should be kept tight.
(C) The positive and negative terminals of the voltmeter and the ammeter should be correctly connected.
(D) The ‘zero error’ in the ammeter and the voltmeter should be noted and taken into consideration while recording the measurements.
(E) The key in the circuit, once plugged in, should not be taken out till all the observations have been completed.
The ‘precautions’ that need to be corrected and revised are
(a) A, C and E
(b) C and E
(c) B and E
(d) A and E
Answer
D
Question. The rest positions of the needles in a milliammeter and voltmeter not in use are as shown in figure A. When a student uses these in his experiment, the readings of the current and the voltage in the experiment as shown in figure B are
(a) 42 mA and 3.2 V
(b) 42 mA and 4.0 V
(c) 34 mA and 3.2 V
(d) 34 mA and 4.0 V
Answer
A
Experiment – 5
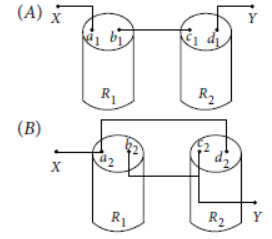
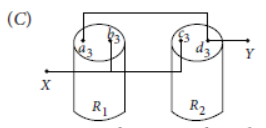
Question. Students A and B connect the two resistors R1 and R2 given to them in the manners shown in the figure.
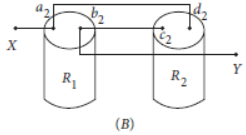
and then insert them at X and Y into the measuring circuit shown below:
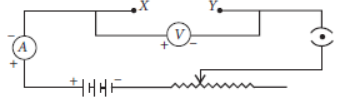
We can then say that
(a) Both the students will determine the equivalent resistance of the series combination of R1 and R2
(b) Both the students will determine the equivalent resistance of the parallel combination of R1 and R2
(c) Student A will determine the equivalent resistance of the series combination while student B will determine the equivalent resistance of the parallel combination of R1 and R2
(d) Student A will determine the equivalent resistance of the parallel combination while student B will determine the equivalent resistance of the series combination of R1 and R2
Answer
C
Question. A student carries out the experiment for studying the dependence of current (I) flowing through a resistor system of R1 and R2 on the potential difference (V) applied to it in different manners as shown.
He connects the terminal marked X and Y to the two free terminals in the circuit given below :
The average value of the ratio V/I of his observations, would then be equal
(a) only in cases A and B
(b) only in cases B and C
(c) only in cases C and A
(d) all the three cases
Answer
B
Question. In the experiment on FInding the equivalent resistance of two resistors connected in series, three students connected the ammeter in their circuits in three ways X, Y and Z shown here.

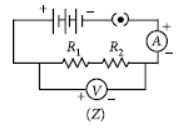
Assuming their ammeters be ideal, the ammeters have been correctly connected in
(a) cases X and Y only
(b) cases Y and Z only
(c) cases X and Z only
(d) all the three cases
Answer
A
Question. A student has correctly set up the circuit for Finding the equivalent resistance of two resistors in parallel. Each terminal of each of the two resistors, in this circuit, would be connected to
(a) only one more components in the circuit
(b) at least two more components in the circuit
(c) at least three more components in the circuit
(d) at least four more components in the circuit
Answer
C
Question. A student sets up her circuit, for finding the equivalent resistance of a series combination of two given resistors R1 and R2 in the manner as shown. She did not obtain the correct results in her experiment because of a mistake in her circuit. This mistake can be corrected by shifting the
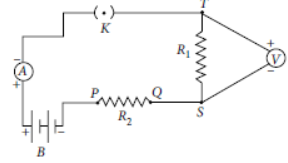
(a) voltmeter and connecting it across P and Q
(b) ammeter and connecting it between K and T
(c) voltmeter and connecting it across T and P with correct polarity
(d) ammeter and connecting it across P and Q
Answer
C
Question. In the experiment on FInding the equivalent resistance of two resistors connected in parallel, three students connected the voltmeter in their circuits in the three ways X, Y and Z as shown here.
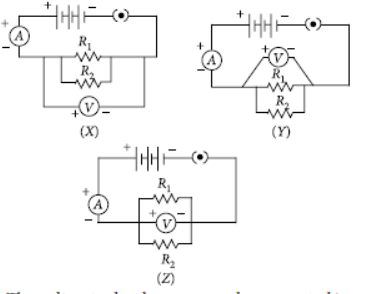
The voltmeter has been correctly connected in
(a) cases X and Y only
(b) cases Y and Z only
(c) cases Z and X only
(d) all the three cases
Answer
D
Question. For three circuits shown here, the same two resistors R1 and R2 have been connected in parallel in all the circuits but the voltmeter and the ammeter have been connected in three different positions. The relation between the three voltmeter and ammeter readings would be
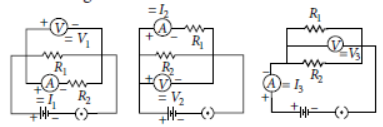
(a) V1 = V2 = V3 and I1 = I2 = I3
(b) V1 ≠ V2 ≠ V3 and I1 = I2 = I3
(c) V1 = V2 = V3 and I1 ≠ I2 ≠ I3
(d) V1 ≠ V2 ≠ V3 and I1 ≠ I2 ≠ I3
Answer
C
Question. In an experiment on finding the equivalent resistance of two resistors, connected in series, a student connects the terminals of the voltmeter to
(a) one terminal of each of the two resistors and these terminals are not interconnected
(b) one terminal of each of the two resistors and these terminals are also interconnected
(c) both the terminals of each of the two resistors
(d) both the terminals of one resistor and one terminal of the other resistor
Answer
A
Question. The values of resistances R1 and R2 marked on the coils are found to be correct. A student arranged the given resistors in the following manner.
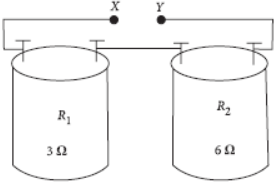
He then connects the terminals marked X and Y to the terminals marked X and Y in the circuit.
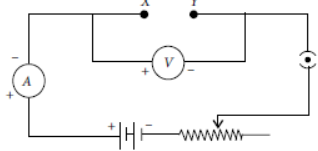
The average value of the ratio V/I in theobservations recorded in the circuit would be
(a) 9 Ω
(b) 6 Ω
(c) 3 Ω
(d) 2 Ω
Answer
A
Experiment – 6
Question. To prepare a good temporary mount of the Petunia leaf peel showing many stomata, the student has to get the peel from the
(a) tip of the leaf
(b) upper surface of the leaf
(c) lower surface of the leaf
(d) point of attachment of the leaf to its petiole.
Answer
C
Question. Four students A, B, C and D, make the records given below, for the parts marked X and Y in this diagram.
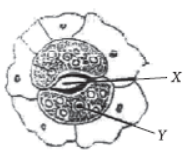
The correct record, out of these, is that of student
(a) A
(b) B
(c) C
(d) D.
Answer
A
Question. The labelling for the slide of leaf peel showing stoma by the four students who made the diagram and tabulated the labels, is as follows:


The student who made the correct labelling is
(a) Student A
(b) Student B
(c) Student C
(d) Student D.
Answer
B
Question. In the diagram of stomata shown below, the labelling of four students was tabulated by the teacher in the table given below. Whose labelling was correct?
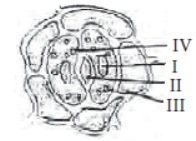
(a) A
(b) B
(c) C
(d) D
Answer
C
Question. A student had drawn the diagram of stomata as shown below in a hurry. He could not be given full marks as he
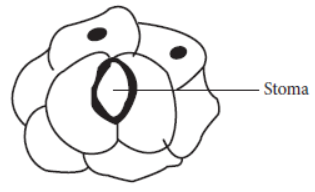
(a) forgot to draw nuclei in guard cells and also to label the diagram
(b) did not draw nuclei in guard cells and other cells
(c) should have drawn nuclei and chloroplasts in guard cells and nuclei in all epidermal cells
(d) did not label the stoma in its correct position.
Answer
C
Question. The temporary mount of the leaf epidermal peel which looked pinkish red under the microscope was
(a) stained in acetocarmine and mounted in glycerine
(b) stained in iodine and mounted in water
(c) stained in safranin and mounted in glycerine
(d) stained in methylene blue and mounted in water.
Answer
C
Question. In the sketch of the stomatal apparatus given below:

Which one of the following is missing?
(a) Cell membranes of the cells
(b) Cell walls of the cells
(c) Nuclei in the guard cells
(d) Chloroplasts in the guard cells
Answer
C
Experiment – 7
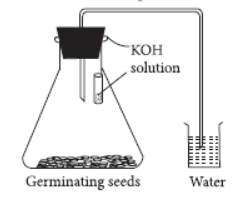
Question.
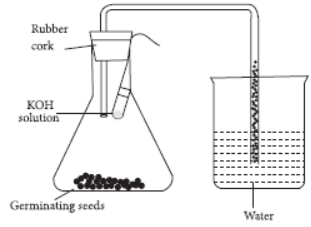
In the experimental setup shown above, KOH solution has been kept in the flask to
(a) react with water to generate oxygen
(b) create a dry atmosphere for wet germinating seeds
(c) absorb carbon dioxide so as to create a partial vacuum
(d) remove impurities present in the air in the flask.
Answer
C
Question. In the following set up which shows that “Carbon dioxide is given out during respiration”, the KOH kept in the flask
(a) makes the air in the flask alkaline
(b) creates partial vacuum in the flask
(c) absorbs moisture present in the flask
(d) provides oxygen for respiration to the germinating seeds.
Answer
B
Question. In the experiment to show that CO2 is given out during respiration, the student uses
(a) lime water
(b) alcohol
(c) KOH solution
(d) iodine solution.
Answer
C
Question. Why is some KOH placed in small test tube in the flask with germinating seeds in the experiment to demonstrate occurrence of respiration in germinating seeds?
(a) To provide oxygen required by the seeds for respiration
(b) To absorb carbon dioxide and create partial vacuum in the flask
(c) To absorb water from the seeds to make them dry
(d) To make the air present in the flask alkaline
Answer
B
Question. The chemical required in the experiment to show that carbon dioxide gas is released during respiration is
(a) potassium biacarbonate
(b) potassium dichromate
(c) potassium permanganate
(d) potassium hydroxide.
Answer
D
Question.
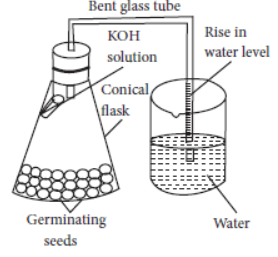
The following precautions were listed for the experimental set-up shown above to prove that carbon dioxide gas is given out during respiration.
A. The stoppered flask should be airtight.
B. The seeds should be immersed in water.
C. There should be a regular supply of oxygen for respiration of seeds.
D. The germinating seeds should be moist.
The correct precautions are
(a) A and B
(b) B and C
(c) C and D
(d) A and D.
Answer
D
Question. Which one of the following is the correct set of three precautions for setting up the experiment to demonstrate that carbon dioxide is evolved during respiration?
(a) Airtight set up; delivery tube dips in water in beaker; flask has seeds which have just germinated
(b) Thread holding KOH test tube; airtight flask; delivery tube above surface of water in the beaker
(c) Germinated seeds under water in the flask; experimental set up not airtight; delivery tube above water level
(d) Delivery tube touching bottom of water; KOH test tube held by a thick wire; seeds covered by water
Answer
A


