See below CBSE Class 10 Science Term 2 Sample Paper Set B with solutions. We have provided CBSE Sample Papers for Class 10 Science as per the latest paper pattern issued by CBSE for the current academic year. All sample papers provided by our Class 10 Science teachers are with answers. You can see the sample paper given below and use them for more practice for Class 10 Science examination.
CBSE Sample Paper for Class 10 Science Term 2 Set B
SECTION – A
1. Name two elements you would expect to show chemical reactions similar to magnesium. What is the basis for your choice?
Ans. Magnesium (Mg) belongs to group 2 known as alkaline earth metals. The two other elements belonging to the same group are calcium (Ca) and strontium (Sr). The basis of choice is the electronic distribution in the valence shell of these elements. All of them have two electrons each in the outermost shell. For example :
K L M N O
Mg (Z = 12) 2 8 2 – –
Ca (Z = 20) 2 8 8 2 –
Sr (Z = 38) 2 8 18 8 2
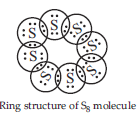
2. What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur? (Hint : The eight atoms of sulphur are joined together in the form of a ring).
Ans. The atomic number (Z) of sulphur is sixteen and its electronic configuration is 2, 8, 6. The sulphur atom has six valence electrons. The chemical formula of sulphur molecule is S8. Each sulphur atom is linked to similar atoms on either sides by single covalent bonds and thus, completes its octet. The molecule is in the form of a ring also represented by crown shape.
3. (a) On which date World AIDS Day is celebrated every year?
(b) Name two sexually transmitted bacterial diseases and their pathogens.
Ans. (a) December 1 is celebrated every year as the world AIDS day to spread information about AIDS among the public.
(b) Two sexually transmitted bacterial diseases are syphilis and gonorrhoea and their pathogens are Treponema pallidum and Neisseria gonorrhoeae respectively.
4. State the basic requirement for sexual reproduction. Write the importance of such reproductions in nature.
Ans. The basic requirement for sexual reproduction is involvement of both sexes, i.e., male and female, to produce an offspring. It takes place by the combination of gametes which come from two different parents. The importance of sexual reproduction in nature are :
(i) Fusion of male and female gametes coming from two different and sexually distinct individuals, exhibit diversity of characters in offspring.
(ii) Meiosis during gametogenesis provides opportunities for new combination of genes, which leads to variation required for evolution and plays a prominent role in the origin of new species. Variations lead to the appearance of such characters, which fit to the changing environment, resulting in the survival of the species.
5. Only variations that confer an advantage to an individual organism will survive in a population. Do you agree with this statement? Why or why not?
Ans. It is not always true. The variations that confer an advantage to an individual organism are definitely of more survival value because natural selection prefers these variations. But there are several other variations which though do not provide advantage to the organism in the present condition, but survive and are inherited to the next generations. Such nonadvantageous variations may become advantageous in future when the environmental conditions change.
OR
Explain the law of purity of gametes.
Ans. Principle of purity of gametes is also known as principle or law of segregation. According to this law, the two unit factors of a character which remain together in an individual do not get mixed up and keep their distinct identity. They separate during gamete formation so that each gamete receives only one factor or gene for each character and is always pure.
6. For a heater rated at 4 kW and 220 V, calculate
(a) the current and the resistance of the heater,
(b) the energy consumed in 2 hours and the cost, if 1 kWh is priced at 50 paise.
Ans. Power, P = 4 kW = 4000 W
Voltage, V = 220 V
Time, t = 2 h
(a) Current, I = P/V = 4000 W / 220 V = 18.2 A
Resistance, R = V/I = 220 V / 18.2 A = 12.1 Ω
(b) Energy consumed = Pt
= 4000 W × 2 h
= 8000 Wh = 8 kWh
Cost = 8 kWh × Rs 0.50/kWh = Rs 4.00
OR
The resistivity of the material of a wire is 44 × 10–8 Ω m. If the resistance of the wire is 14 Ω and its diameter is 1 mm, calculate the length of the wire.
Ans.
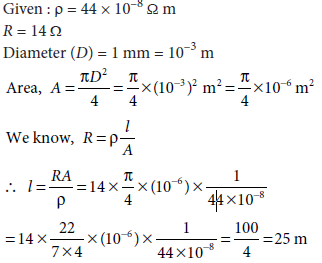
7. In natural conditions food chains never operate as an isolated sequence, but are interconnected with each other. In other words, when different types of organisms at different trophic levels, instead of forming a linear chain, remain interconnected with each other, it forms a complex pathway rather than a simple chain.
(a) Draw any food chain operating in grassland ecosystem.
(b) Why decomposers are also called reducers and micro-consumers?
Ans. (a) Grass → Grasshopper → Frog → Snake → Eagle
(b) Decomposers are also called as reducers because they are able to remove or degrade the dead bodies of organisms and due to their small size they are known as micro-consumers.
OR
How does the use of recycled paper help in protecting the environment?
Ans. Recycling is the process of converting waste materials into reusable objects to prevent waste of potentially useful materials. Thus, it reduces the consumption of fresh raw materials, energy, air and water pollution. E.g., recycling of paper, glass, plastic, etc.
Paper recycling is done at industry level, school and at home also. Recycled paper is developed by processing of all the waste paper materials collected from different places. Some advantages are :
(i) If we recycle paper, we can reduce the number of trees that are cut to produce papers.
(ii) The garbage constituted by paper can be reduced to a considerable amount.
(iii) Level of pollution can be decreased as papers are biodegradable and there will be less accumulation if papers are recycled.
SECTION – B
8. Compare and contrast three differences on the arrangement of elements in Mendeleev’s periodic table and the modern periodic table.
Ans. (i) In Mendeleev’s periodic table the elements are arranged in the increasing order of atomic masses. On the other hand, in the Modern Periodic Table the elements are arranged in the increasing order of atomic numbers which is more fundamental property than the atomic mass.
(ii) In the Mendeleev’s periodic table, the position of the elements was in accordance with atomic mass. In the Modern Periodic Table the position of the elements is governed by electronic configurations, which determine their properties.
(iii) In Mendeleev’s periodic table, the position of isotopes was not justified. In Modern Periodic Table, the classification is based on the atomic number and not atomic mass and hence the position of isotopes is fully justified.
(iv) Mendeleev’s periodic table had some anomalies on the basis of atomic mass. In Modern Periodic Table, there are no misfits in terms of atomic number. For example, in Mendeleev’s periodic table, potassium was having position lower than argon. But in Modern Periodic Table, this has been solved. Argon precedes potassium because argon has atomic number 18 and potassium has 19.
(v) The Mendeleev’s periodic table does not explain satisfactorily as to why the properties of the elements are repeated at regular intervals of 2, 8, 18 and 32. The Modern Periodic Table explains this logically. (any three)
9. Elements forming ionic compounds attain noble gas electronic configuration by either gaining or losing electrons from their valence shells. Explain giving reason why carbon cannot attain such a configuration in this manner to form its compounds. Name the type of bonds formed in ionic compounds and in the compounds formed by carbon. Also explain with reason why carbon compounds are generally poor conductors of electricity.
Ans. Ionic compounds are formed either by gaining or losing electrons from the outermost shells, but carbon, which has four electrons in its outermost shell, cannot form ionic bonds because :
1. If carbon forms ionic bonds by gaining four electrons to attain a noble gas configuration then it would be difficult for six protons in the nucleus to hold ten electrons.
2. If carbon forms ionic bonds by loss of four electrons then it would require a lot of energy to remove these electrons from outermost shell.
Due to these reasons, carbon forms covalent bonds by sharing the valence electrons.
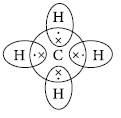
Type of bonds formed in ionic compounds are called electrovalent bonds and the type of bonds formed in carbon compounds are called covalent bonds. Covalent bonds are those bonds which are formed by sharing the valence electrons between two atoms. Electron dot structure of methane is shown in the figure. Covalent compounds are generally poor conductors of electricity because they do not have free electrons or ions.
OR
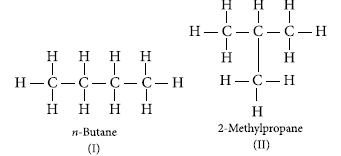
Explain isomerism. State any four characteristics of isomers. Draw the structures of possible isomers of butane, C4H10.
Ans. Isomers are those compounds which have same molecular formula but different structures i.e., show different physical properties. The phenomenon of existing into isomers is called isomerism. Four characteristics of isomers are :
(i) They have same molecular formula but different structures.
(ii) Isomers are possible only for those hydrocarbons which have four or more carbon atoms.
(iii) Due to isomerism, a given molecular formula can represent two or more different compounds.
(iv) Due to isomerism, the different compounds have different properties.
The structures of possible isomers of butane (C4H10) are :
10. Mendel crossed homozygous round and yellow seeded pea plant with homozygous wrinkled and green seeded pea plants for two generations. Name this cross and identify the Mendel’s postulate that is proved by this cross.
Ans. The given cross represents the dihybrid cross.
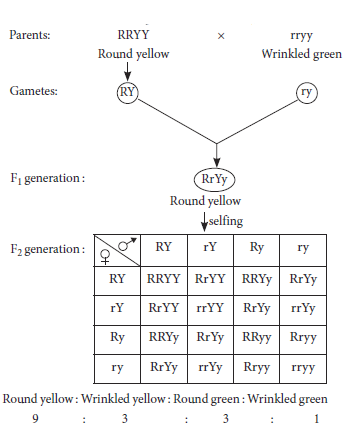
Dihybrid cross proves Mendel’s second law’ “law of independent assortment”. According to this law, genes of different characters located on different pairs of chromosomes segregate independently of one another during gamete formation.
11. (a) What is electromagnetic induction?
(b) State the rule which gives the direction of induced current.
Ans. (a) Electromagnetic induction is the phenomenon in which an electric current is induced in a circuit because of a changing magnetic field.
(b) Direction of induced current is given by Fleming’s right-hand rule. If the forefinger, middle finger and thumb of the right hand are stretched at right angles to each other, with the forefinger in the direction of the field and the thumb in the direction of the motion of the wire then the induced current in the wire is in the direction of the middle finger.
12. Why does a current-carrying conductor kept in a magnetic field experience force ? On what factors does the direction of this force depend ?
Ans. The force on a current-carrying conductor in a magnetic field is due to interaction between magnetic field due to current carrying conductor and the external magnetic field in which the conductor is placed.
The direction of the force acting on the current carrying conductor placed in the magnetic field depends upon
(i) direction of the current through the conductor and
(ii) direction of the magnetic field in which the conductor is placed.
OR
Name and state the rule used for determination of direction of magnetic force.
Ans. The direction of the force acting on the current carrying conductor placed in the magnetic field is determined by using Fleming’s left hand rule. According to this rule, stretch the thumb, forefinger and middle finger of your left hand such that they are mutually perpendicular. If the fore finger in the direction of magnetic field and the middle finger in the direction of current, then the thumb will point in the direction of motion or force acting on the conductor.
13. Name any one greenhouse gas and its possible source of production on a large scale. What are the harmful effects of it?
Ans. Major greenhouse gases are CO2-60%, CH4-20%, CFCs-14% and N2O-6%. Out of which CO2 is present in maximum amount. Source of CO2 is burning of fossil fuels, volcanic eruptions and respiration process. Due to increased level of CO2 in the atmosphere, global atmospheric temperature during the past century has increased to 0.6%. It is called global warming, which results in melting of polar ice caps and rise in sea level, etc.
SECTION – C
This section has 02 case-based questions (14 and 15). Each case is followed by 03 sub-questions (a, b and c). Parts a and b are compulsory. However, an internal choice has been provided in part c.
14. All flowering plants show sexual reproduction. Sexual reproduction is the process of development of new organisms through the formation and fusion of gametes. The diversity of structures of the flowers and floral parts shows an amazing range of adaptation to ensure formation of the end products of sexual reproduction, the fruits and seeds.
(a) What happens to the zygote after fertilization in flowering plants?
(b) What is germination?
(c) Identify the labelled parts A, B and C of the given figure.

Ans. (a) After fertilization, the zygote divides several times to form an embryo within the ovule. The ovule develops a tough coat and is gradually converted into a seed. The ovary grows rapidly and ripens to form a fruit. Meanwhile, the petals, sepals, stamens, style and stigma may shrivel and fall off.
(b) Germination is the process involving all changes that take place from the time when a dry, viable seed starts to grow when placed under suitable condition of germination to the time when the seedling becomes established on the substratum.
(c) A – Plumule, B – Radicle, C – Cotyledon
OR
Identify the labelled parts that develop into stem and root respectively.
Ans. Labelled parts A and B represent plumule and radicle that develop into stem and root respectively.
15. You are given following current-time graphs (a) and (b) from two different sources. Give the following answers.
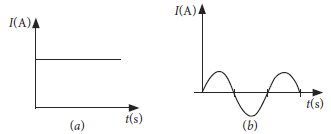
(a) Identify any one source for each type of these currents.
(b) What is the frequency of current in case (b) in India?
(c) Use graphs to write two differences between the currents in two cases.
Ans. (a) Cell or a battery – Source of DC.
Generator – Source of AC.
(b) Frequency of AC is 50 Hz in India while DC has zero frequency.
(c) In case (a), current remains constant and frequency is zero whereas in case (b). In case (a), direction of current not change from negative to positive.
OR
Name the type of current in two cases. After what interval of time current in the case (b) changes its direction
Ans. DC in case (a) and AC in the case (b). From graph, the time interval after which AC current changes its direction is 0.01 second.

