Please refer to MCQ Questions Chapter 9 Coordination Compounds Class 12 Chemistry with answers provided below. These multiple-choice questions have been developed based on the latest NCERT book for class 12 Chemistry issued for the current academic year. We have provided MCQ Questions for Class 12 Chemistry for all chapters on our website. Students should learn the objective based questions for Chapter 9 Coordination Compounds in Class 12 Chemistry provided below to get more marks in exams.
Chapter 9 Coordination Compounds MCQ Questions
Please refer to the following Chapter 9 Coordination Compounds MCQ Questions Class 12 Chemistry with solutions for all important topics in the chapter.
MCQ Questions Answers for Chapter 9 Coordination Compounds Class 12 Chemistry
Question. The oxidation states of Cr, in [Cr(H2O)6 ]Cl3, [Cr(C6H6 )2 ], and K2[Cr(CN)2(O)2(O2 )(NH3 )] respectively are
(a) +3, +4 and +6
(b) +3, +2 and +4
(c) +3, 0 and +6
(d) +3, 0 and +4
Answer
C
Question. The number of geometrical isomers that can exist for square planar [Pt (Cl) (py) (NH3 ) (NH2OH)]+ is (py = pyridine)
(a) 2
(b) 3
(c) 4
(d) 6
Answer
B
Question. Consider the following complexes,
1. K2PtCl6
2. Pt Cl4 2NH3
3. PtCl4 3NH3
4. PtCl4 5NH3
Their respective electrical conductances in aqueous solutions are
(a) 256, 0, 97, 404
(b) 404, 0, 97, 256
(c) 256, 97, 0, 404
(d) 404, 97, 256, 0
Answer
A
Question. The coordination number and the oxidation state of the element E in the complex [E(en)2(C2O4) ]NO2 are respectively, [where, en is ethylene diamine]
(a) 6 and 2
(b) 4 and 2
(c) 4 and 3
(d) 6 and 3
Answer
D
Question. Consider the following reaction and statements :
[Co(NH3 )4Br2 ]+ + B−→ [Co(NH3 )3 Br3 ] + NH3
I. Two isomers are produced if the reactant complex ion is a cis-isomer.
II. Two isomers are produced if the reactant complex ion is a trans-isomer.
III. Only one isomer is produced if the reactant complex ion is a trans-isomer.
IV. Only one isomer is produced if the reactant complex ion is a cis-isomer.
The correct statements are
(a) (I) and (II)
(b) (I) and (III)
(c) (III) and (IV)
(d) (II) and (IV)
Answer
B
Question. The magnetic moment of the complex anion
[Cr(NO) (NH3 ) (CN)4 ]2− is
(a) 5.91 BM
(b) 3.87 BM
(c) 1.73 BM
(d) 2.82 BM
Answer
C
Question. IUPAC name of [Pt (NH3 )2 Cl (NO2 )] is
(a) platinum diamminechloronitrite
(b) chloronitrito-N-ammineplatinum (II)
(c) diamminechloridonitrito-N-platinum (II)
(d) diamminechloronitrito-N-platinate (II)
Answer
C
Question. Which among the following will be named as dibromidobis- (ethylenediamine) chromium (III) bromide?
(a) [Cr(en)3 ]Br3
(b) [Cr(en)2 Br2 ]Br
(c) [Cr(en)Br4 ]–
(d) [Cr(en)Br2 ]Br
Answer
B
Question. If excess of AgNO3 is added to 100 mL of a 0.024 M solution of dichlorobis (ethylene diamine) cobalt (III) chloride how many moles of AgCl be precipitated?
(a) 0.0012
(b) 0.0016
(c) 0.0024
(d) 0.0048
Answer
C
Question. The number of geometrical isomers of the complex [Co(NO2 )3(NH3 )3 ] is
(a) 2
(b) 4
(c) 3
(d) 0
Answer
A
Question. The complex, [Pt(py)(NH3 )BrCl] will have how many geometrical isomers?
(a) 2
(b) 3
(c) 4
(d) 0
Answer
B
Question. In which of the following complexes the crystal field splitting will be least?
(a) [Fe(H2O)6 ]3−
(b) [Cr(NH3)6 ]3+
(c) [Co(C2O4 )3 ]3−
(d) Ni(CO)4
Answer
A
Question. Which one of the following complexes shows optical isomerism?
(a) cis [Co(en)2 Cl2 ]Cl
(b) trans [Co(en)2 Cl2 ]Cl
(c) [Co(NH3)4 Cl2]Cl
(d) [Co(NH3 )3 Cl ]3
Answer
C
Question. Which of the following complex species is not expected to exhibit optical isomerism?
(a) [Co(en)2 Cl2 ]+
(b) [Co(NH3 )3 Cl3 ]
(c) [Co(CN)(NH3)2 Cl ]2+
(d) [Co(en)3]3+
Answer
B
Question. Which one of the following has an optical isomer?
(en = ethylenediamine)
(a) [Zn(en)(NH3 )2 ]2+
(b) [Co(en)3]3+
(c) [Co(H2O)4 (en)3+]3+
(d) [Zn(en)2 ]2+
Answer
B
Question. Type of isomerism which exists between
[Pd (C6H5 )2(SCN)2 ] and [Pd(C6H5 )2(NCS)2 ] is
(a) linkage isomerism
(b) coordination isomerism
(c) ionisation isomerism
(d) solvate isomerism
Answer
A
Question. Which of the following pairs represents linkage isomers?
(a) [Cu(NH3)4 ][PtCl4 ] and [Pt(NH3)4 ][CuCl4]
(b) [Pd(PPh3 )2 (NCS)2 ] and [Pd(PPh3 )2 (SCN)2 ]
(c) [Co(NH3 )5 NO3 ]SO4 and [Co(NH3 )5 SO ]NO3
(d) [PtCl2 (NH3)4 ]Br and [PtBr2 (NH3 )4 ]Cl2
Answer
B
Question. The IUP AC name of K2[PtCl6 ] is
(a) hexachloroplatinate potassium
(b) potassium hexachloroplatinate (IV)
(c) potassium hexachloroplatinate
(d) potassium hexachloroplatinum (IV)
Answer
B
Question. Consider the following complex ions, P,Q and R.
P = [FeF6 ]3- ,Q= [V(H2O)6 ]2+ and
R=[Fe(H2O)6 ]2+.
The cotTect order of the complex ions, according to their spin-only magnetic moment values
(in BM) is
(a) R < Q < P
(b) Q < R < P
(c) R < P < Q
(d) Q < P < R
Answer
B
Question. NiCl2 [P(C2H5)2 (C2H5)2 exhibit temperature dependen t magnetic behaviour (paramagnetic / diamagnetic). The coordination geometries of Ni2+ in the paramagnetic and diamagnetic states are respectively
(a) tetrahedral and tetrahedral
(b) square planar and square planar
(c) tetrahedral and square planar
(d) square planar and tetrahedral
Answer
C
Question. What is the magnetic moment of[FeF6 ]3- ?
(a) 2 BM
(b) 5.9 BM
(c) 7 BM
(d) 35 BM
Answer
B
Question. In [NiCl4 ]2- , the type of hybridisation for Ni is
(a) sp3d2
(b) dsp3
(c) sp3
(d) dsp2
Answer
C
Question. Maximum value of paramagnetism is shown by
(a) [Fe(CN)6]3-
(b) [Cr(CN)6]3-
(c) [Co(CN)6]3-
(d) [Sc(CN)6]3-
Answer
B
Question. Geometrical shapes of the complexes formed by the reaction of Ni2+ with Cl– , CN– and H2O, respectively, are
(a) octahedral, tetrahedral and square planar
(b) tetrahedral, square planar and octahedral
(c) square planar, tetrahedral and octahedral
(d) octahedral, square planar and octahedral
Answer
B
Question. Among the following complexes (K-P), K3 [Fe(CN)6 ] (K), [Co(NH3)6]Cl3 (L), Na3[Co(ox)3 ] (M)
[Ni(H2O)6]Cl2 (N), K2 [Pt(CN)4 ] (O) and [Zn(H2O)6 ](NO3 )2 (P) the diamagnetic complexes are
(a) K ,L ,M ,N
(b) K ,M ,O ,P
(c) L ,M ,O ,P
(d) L ,M ,N ,O
Answer
C
Question. Which of the following facts about the complex [Cr(NH3)6 ]Cl3 is wrong?
(a) The complex involves d 2sp3 hybridisation and is octahedral in shape
(b) The complex is paramagnetic
(c) The complex is an outer orbital complex
(d) The complex gives white precipitate with silver nitrate solution
Answer
C
Question. The magnetic moment (spin only) of [NiCl4 ]2- is
(a) 1.82 BM
(b) 5.46 BM
(c) 2.82 BM
(d) 1.41 BM
Answer
C
Question. Among the ligands NH3, en, CN– and CO, the correct order of their increasing field strength, is
(a) CO < NH3 < en < CN–
(b) NH3 < en < CN– < CO
(c) CN–<NH3 < CO < en
(d) en < CN– < NH3 < CO
Answer
B
Question. The complex ion which has the highest magnetic moment among the following is
(a) [CoF6]3-
(b) [Co(NH3)6]3+
(c) [Ni(NH3)4 ]2+
(d) [Ni(CN)4 ]2-
(e)[Fe(CN)6]4-
Answer
A
Question. The correct statement with respect to the complexes Ni(CO)4 and [Ni(CN)4 ]2- is
(a) nickel is in the same oxidation state in both
(b) both have tetrahedral geometry
(c) both have square planar geometry
(d) have square planar and tetrahedral geometry respectively
(e) have tetrahedral and square planar geometry respectively
Answer
E
Question. The complex showing a spin-only magnetic moment of 2.82 BM is
(a) Ni(CO)4
(b) [NiCl4 ]2-
(c) Ni(PPh3)4
(d) [Ni(CN)4 ]2-
Answer
B
Question. Which one of the following complex is an outer orbital complex?
(Atomic numberofMn = 25, Fe = 24, Co = 27, Ni = 28)
(a) [Fe(CN)6]4-
(b) [Mn(CN)6]4-
(c) [Co(NH3)6]3+
(d) [Ni(NH3)6]2+
Answer
D
Question. Which one of the following is wrongly matched
(a) [Cu(NH3)4 ]2+ – Square planar
(b) [Ni(CO)4 ] – Neutral ligand
(c) [Fe(CN6)]3- – sp3d1
(d) [Co(en)3 ]3+ – Follows EAN rule
Answer
C
Question. Show the coordination number of the metal ion, its oxidation number, the number of electrons in d-orbitals and the number of unpaired electrons in d-orbitals respectively in complex [Co(H2O)4SO3]CI.
(a) 6, 3, 6, 4
(b) 6, 3, 6, 0
(c) 5, 3, 6, 4
(d) 5, 3, 6, 0
Answer
C
Question. [Sc(H2O)6 ]3+ ion is
(a) colourless and diamagnetic
(b) coloured and octahedral
(c) colourless and paramagnetic
(d) coloured and paramagnetic
Answer
A
Question. In spectrochemical series chlorine is above than water, i.e., Cl > H2O, this is due to
(a) good π-acceptor properties of Cl
(b) strong σ-donor and good π-acceptor properties of Cl
(c) good π-donor properties of Cl
(d) larger size of Cl than H2O
Answer
B
Question. Match the following columns.
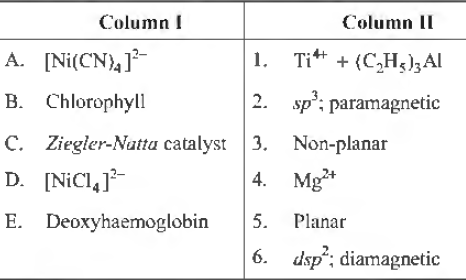
Codes
A B C D
(a) 6 4 1 2
(b) 2 4 1 6
(c) 2 4 1 6
(d) 6 4 1 2
(e) 2 4 3 6
Answer
A
Question. Which of the following species will be diamagnetic ?
(a) [Fe(CN)6]
(b) [FeF6]3-
(c) [Co(C2O4)3 ]3-
(d) [CoF6]3-
Answer
A
Question. The structure of which of the following chloro species can be explained on the basis of dsp2 hybridisation?
(a) PdCl42−
(b) FeCl42−
(c) CoCl42−
(d) NiCl42−
Answer
A
Question. Both [Ni(CO4] and [Ni(CN4)]2− are diamagnetic. The hybridisations of nickel in these complexes are respectively,
(a) sp3 ,sp3
(b) sp2 , dsp3
(c) dsp3 , sp
(d) dsp2 , dsp2
Answer
B
Question. Which of the following facts about the comple [Cr(NH3 )6 ]Cl3 is wrong?
(a) The complex involves d2sp3 hybridisation and is octahedral in shape
(b) The complex is paramagnetic
(c) The complex is an outer orbital complex
(d) The complex gives white precipitate with silver nitrate solution
Answer
C
Question. Which of the following is diamagnetic ?
(a) [Fe(CN)6 ]3−
(b) [Co(ox)3 ]3−
(c) [FeF6]3−
(d) [CoF6 ]3−
Answer
B
Question. The magnetic moment (spin only) of [NiCl4]2− is
(a) 1.82 BM
(b) 5.46 BM
(c) 2.82 BM
(d) 1.41 BM
Answer
C
Question. In which of the following octahedral complex species the magnitude of Δo will be maximum?
(a) [Co (H2O6) ]2+
(b) [Co (CN)6 ]3−
(c) [Co(C2O4)3 ]3−
(d) [Co (NH3)6 ]3+
Answer
B
Question. A solution containing 2.675 g of CoCl3 6NH3 (molar mass = 267.5 g mol−1) is passed through a cation exchanger. The chloride ions obtained in solution were treated with excess of AgNO3 to give 4.78 g of AgCl (molar mass = 143.5 g mol−1 ) . The formula of the complex is (Atomic mass of Ag = 108 u)
(a) [Co(NH3)6 ]Cl3
(b) [CoCl2 (NH3)4]Cl
(c) [CoCl3(NH3)3]
(d) [CoCl(NH3)5]Cl2
Answer
A
Question. On treatment of 100 mL of 0.1 M solution of CoCl3.6H2O with excess of AgNO3;1.2 × 1022 ions are precipitated. The complex is
(a) [Co(H2O)4Cl2] Cl H2O
(b) [Co(H2O)3Cl3 ].3H2O
(c) [Co(H2O)6]Cl3
(d) [Co(H2O)5Cl] Cl2 .H2O
Answer
D
Question. According to EAN rule, how many CO groups should be attached to Fe?
(a) 4
(b) 5
(c) 6
(d) 8
Answer
B
Question. The value of CFSE for complex ion [CoCl6]4− is 18000 cm−1. The CFSE for [CoCl4]2− complex ion will be
(a) 18000 cm−1
(b) 16000 cm−1
(c) 8000 cm−1
(d) 2000 cm−1
Answer
C
Question. Which of the following compounds is not yellow coloured?
(a) Zn2 [Fe(CN)2]
(b) K3 [Co(NO2)6]
(c) (NH4)3 [As (Mo3O10)4]
(d) BaCrO4
Answer
A
Question. The complex [Fe(H2O)5NO]2+ is formed in the brown ring test for nitrates when freshly prepared FeSO4 solution is added to aqueous solution of NO3− followed by addition of conc. H2SO4. Select the correct statement about this complex.
(a) Colour change is due to charge transfer
(b) It has iron in +1 oxidation state and nitrosyl as NO+
(c) It has magnetic moment of 3.87 BM confirming three unpaired electrons in Fe
(d) All the above are correct statements
Answer
D
Question. Among the following which is not the p-bonded organometallic compound?
(a) K[PtCl3 ( n2 — C2H4)]
(b) Fe( n5 — C5H5 )
(c) (CH3)4 Sn
(d) Cr( n6 —C6H6 )2
Answer
C
Question. The colour of the coordination compounds depend on the crystal field splitting. What will be the correct order of absorption of wavelength of light in the visible region, for the complexes, [Co(CN)6]3−, [Co(CN)6 ]3−, [Co(H2O)6]3+
(a) [Co(CN)6 ]3− > [Co(NH3)6]3+ > [Co(CN)6]3+
(b) [Co(CN3)6 ]3+ > [Co(NH2O)6]3+ > [Co(CN)6]3+
(c) [Co(H2O)6 ]3+ > [Co(NH3)6]3+ > [Co(CN)6]3+
(d) [Co(CN)6 ]3− > [Co(NH3)6]3+ > [Co(H2O)6]3+
Answer
C
Question. Ferrocene is an example of
(a) sandwiched complex
(b) pi-bonded complex
(c) a complex in which all the five carbon atoms of cyclopentadiene anion are bonded to the metal
(d) All of the above
Answer
D
Question. For an octahedral complex, which of the following d- electron configuration will give maximum CFSE ?
(a) High spin d6
(b) Low spin d4
(c) Low spin d5
(d) High spin d7
Answer
C
Question. Among the following metal carbonyls, the C—O bond order is lowest in
(a) [Mn(CO)6]+
(b) [Fe(CO)5]+
(c) [Cr(CO)6]+
(d) [V(CO)6]−
Answer
B
Question. Which of the following statements is incorrect ?
(a) Fe3+ ion also gives blood red colour with SCN− ion
(b) Fe3+ ion gives red colour with SCN− ion
(c) On passing H2S into Na2ZnO solution, a white ppt of ZnS is formed
(d) Cupric ion reacts with excess of ammonia solution to give deep blue colour of [Cu (NH3)4]2+ ion
Answer
A
Question. The stabilisation of coordination compounds due to chelation is called the chelate effect. Which of the following is the most stable complex species?
(a) [Fe(CO)5]
(b) [Fe(CN)6]3−
(c) [Fe(C3O4)3]3−
(d) [Fe(H2O6]3+
Answer
C
Question. Among the ligands NH3 , en,CN− and CO, the correct order of their increasing field strength, is
(a) CO < NH3 < en < CN−
(b) NH3 < en < CN− < CO
(c) CN− < NH3 < CO < en
(d) en < CN− < NH3 < CO
Answer
B
Question. An aqueous solution of an inorganic salt (X ), when added to an aqueous solution of barium chloride, a precipitate insoluble in dil. HCl is obtained. Addition of excess of KI to X gives a brown precipitate which turns white on addition of excess of hypo. With an aqueous solution of potassium ferrocyanide, a chocolate coloured precipitate is formed. X is
(a) Cu (NH3)4SO4
(b) CuSO4 5H2O
(c) ZnSO4 5H2O
(d) AgNO3
Answer
B
Question. The octahedral complex of a metal ion M3+ with four monodentate ligands L1,L2,L3 and L4 absorb wavelengths in the region of red, green, yellow and blue, respectively. The increasing order of ligand strength of the four ligands is
(a) L4 < L3 < L2 < L1
(b) L1 < L3 < L2 < L4
(c) L3 < L2 < L4 < L1
(d) L1 < L2 < L4 < L3
Answer
D
Question. Which of the following compounds shows optical isomerism?
(a) [Co(CN)6]3−
(b) [Cr(C2O3) ]3+
(c) [ZnCl4]2−
(d) [Cu(NH3)4]2+
Answer
B
Question. The equation which is balanced and represents the correct product(s) is
(a) Li2O + 2KCl → 2LiCl +K2O
Question. b) [CoCl (NH3)5+ + 5H+ → CO2+ + 5NH+4 + Cl−
(c) [Mg (H2O6 )2+ + (EDTA )4− →Excess NaOH [Mg (EDTA)]2+ + 6H2O
(d) CuSO4 + 4KCN → K2 Cu(CN)4 K2SO4
Answer
B
Question. The correct order for the wavelength of absorption in the visible region is
(a) [Ni(NO2)6]4− < [Ni(NH3)6]2+ < [Ni(H2O6)]2+
(b) [Ni(NO2)6]4− < [Ni(N2O)6]2+ < [Ni(H3O6)]2+
(c) [Ni(NO2)6]2+ < [Ni(NH3)6]2+ < [Ni(H2O6)]4−
(d) [Ni(NO3)6]2+ < [Ni(N2O)6]2+ < [Ni(H2O6)]4−
Answer
A
Question. Which can exist both as diastereomer and enantiomer?
(a) [Pt(en)3]4+
(b) [Pt(en)2 ClBr]2+
(c) [Ru(NH3)4 Cl2]0
(d) [PtCl2Br2 ]0
Answer
B
Question. What is the ratio of uncomplexed to complexed Zn2+ ion in a solution that is 10 M in NH3, if the stability constant of [Zn(NH3)4]2+ is 3 × 109?
(a) 3.3 × 10−9
(b) 3.3 × 10−11
(c) 3.3 × 10−14
(d) 3 × 10−13
Answer
C
Question. How many moles of AgCl would be obtained, when 100 mL of 0.1 M Co(NH3 )5Cl3 is treated with excess of AgNO3?
(a) 0.01
(b) 0.02
(c) 0.03
(d) None of these
Answer
B
Question. A complex is prepared by mixing CoCl3 and NH3 in the molar ratio1: 4. 0.1 Msolution of this complex was found to freeze at −0.372° C. What is the formula of the complex ? [K (water) 1.86 C /m] f = °
(a) [Co(NH3)4Cl2]Cl
(b) [Co(NH3)5Cl]Cl2
(c) [Co(NH3)3Cl3]Cl
(d) [[Co(NH3)6Cl]Cl3
Answer
A
Question. Ammonia forms the complex ion [Cu(NH3)4]2+ with copper ions in the alkaline solutions but not in acidic solutions. What is the reason for it ?
(a) In acidic solutions hydration protects copper ions
(b) In acidic solutions protons coordinate with ammonia molecules forming NH4+ ions and NH3 molecules are not available
(c) In alkaline solutions insoluble Cu(OH)2 is precipitated which is soluble in excess of any alkali
(d) Copper hydroxide is an amphoteric substance
Answer
B

We hope you liked the above provided MCQ Questions Chapter 9 Coordination Compounds Class 12 Chemistry with solutions. If you have any questions please ask us in the comments box below.


