Please refer to Assignments Class 12 Chemistry General Principles and Processes of Isolation of Elements Chapter 6 with solved questions and answers. We have provided Class 12 Chemistry Assignments for all chapters on our website. These problems and solutions for Chapter 6 General Principles and Processes of Isolation of Elements Class 12 Chemistry have been prepared as per the latest syllabus and books issued for the current academic year. Learn these solved important questions to get more marks in your class tests and examinations.
General Principles and Processes of Isolation of Elements Assignments Class 12 Chemistry
Question. In the blast furnace iron oxide is reduced by
(a) silica
(b) CO
(c) carbon
(d) limestone
Answer
B
Question. The natural materials from which an element can be extracted economically are called
(a) ores
(b) minerals
(c) gangue
(d) None of these
Answer
A
Question. When a metal is to be extracted from its ore and the gangue associated with the ore is silica, then
(a) an acidic flux is needed
(b) a basic flux is needed
(c) both acidic and basic fluxes are needed
(d) Neither of them is needed
Answer
B
Question. Which of the following is chalcopyrite?
(a) CuFeS2
(b) FeS2
(c) KMgCl3.6H2O
(d) Al2O3.2H2O
Answer
A
Question. Haematite is the ore of
(a) Pb
(b) Cu
(c) Fe
(d) Au
Answer
C
Question. Hydro-metallurgical process of extraction of metals is based on
(a) complex formation
(b) hydrolysis
(c) dehydration
(d) dehydrogenation
Answer
A
Question. Which one of the following is a mineral of iron ?
(a) Malachite
(b) Cassiterite
(c) Pyrolusite
(d) Magnetite
Answer
D
Question. The impurities associated with mineral used in metallurgy are called collectively?
(a) Slag
(b) Flux
(c) Gangue
(d) Ore
Answer
C
Question. Brine is electrolysed by using inert electrodes. The reaction at anode is ________.
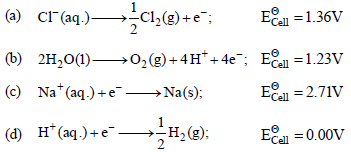
Answer
A
Question. In froth flotation process many chemicals (frother , collector, activator, and depressant) are used. Which of the following is a frother ?
(a) CuSO4
(b) NaCN+ alkali
(c) Pine oil
(d) Potassium xanthate
Answer
C
Question. The most abundant element in the earth’s crust (by weight) is
(a) Si
(b) Al
(c) O
(d) Fe
Answer
C
Question. ΔG° vs T plot in the Ellingham’s diagram slopes downward for the reaction
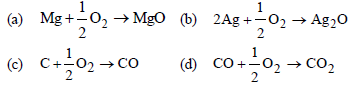
Answer
C
Question. Malachite is an ore of
(a) iron
(b) copper
(c) mercury
(d) zinc
Answer
B
Question. General method for the extraction of metal from oxide ore is
(a) carbon reduction
(b) reduction by aluminium
(c) reduction by hydrogen
(d) electrolytic reduction
Answer
A
Question. Which of the following reaction represents calcination process ?

Answer
B
Question. According to Ellingham diagram, the oxidation reaction of carbon to carbon monoxide may be used to reduce which one of the following oxides at the lowest temperature ?
(a) Al2O3
(b) Cu2O
(c) MgO
(d) ZnO
Answer
B
Question. Matrix is defined as –
(a) the unwanted foreign material present in the ore
(b) the flux added to remove the unwanted impurities from ore
(c) the slag formed as a result of the reaction of flux with gangue
(d) the material used in the reduction of metal oxide to metal
Answer
A
Question.Froth floatation process is used for the metallurgy of
(a) chloride ores
(b) amalgams
(c) oxide ores
(d) sulphide ores
Answer
D
Question. While extracting an element from its ore, the ore is grounded and leached with dil. potassium cyanide solution to form the soluble product potassium argento cyanide. The element is
(a) Lead
(b) Chromium
(c) Manganese
(d) Silver
Answer
D
Question. Which of the following reactions taking place in the blast furnace during extraction of iron is endothermic?
(a) CaCO3 → CaO + CO2
(b) 2C + O2 → 2CO
(c) C + O2 → CO2
(d) Fe2O3 + 3CO → 2Fe + 3CO2
Answer
A
Question. Leaching is a process of
(a) reduction
(b) concentration
(c) refining
(d) oxidation
Answer
B
Question. Ellingham diagram normally consists of plots of
(a) ΔSº vs T
(b) ΔfGº vs ΔSº
(c) ΔGº vs T
(d) ΔHº vs ΔT
Answer
C
Question. Which one of the following ores is concentrated by chemical leaching method?
(a) Galena
(b) Copper pyrite
(c) Cinnabar
(d) Argentite
Answer
D
Question. Electromagnetic separation is used in the concentration of
(a) copper pyrites
(b) bauxite
(c) cassiterite
(d) cinnabar
Answer
C
Question. For which ore of the metal, froth floatation method is used for concentration?
(a) Horn silver
(b) Bauxite
(c) Cinnabar
(d) Heamatite
Answer
C
Question. Cassiterite is an ore of
(a) Mn
(b) Ni
(c) Sb
(d) Sn
Answer
D
Question. Which one of the following ores is not concentrated by froth floatation process?
(a) Copper pyrites
(b) Pyrargyrite
(c) Pyrolusite
(d) Zinc blende
Answer
C
Question. What is anode mud?
(a) Fan of anode
(b) Metal of anode
(c) Impurities collected at anode in electrolysis during purification of metals
(d) All of these
Answer
C
Question. Froth flotation process is based on
(a) wetting properties of ore particle
(b) specific gravity of ore particles
(c) magnetic properties of ore particles
(d) electrical properties of ore particles
Answer
A
Question. In the froth flotation process of concentration of ores, the ore particles float because they:
(a) are light
(b) are insoluble
(c) have the surface which is not wetted easily
(d) have a constant electrical charge
Answer
C
Question. Which of the following is an ore of tin ?
(a) Carborundum
(b) Epsomite
(c) Cassiterite
(d) Spodumene
Answer
C
Question. Main function of roasting is
(a) to remove volatile substances
(b) oxidation
(c) reduction
(d) slag formation
Answer
A
Question. The furnace used to prepare commercial iron is lined with which of the following ?
(a) Haematite
(b) Magnetite
(c) Ironpyrites
(d) Both (a) and (b)
Answer
A
Question. Heating of pyrites in air for oxidation of sulphur is called
(a) roasting
(b) calcination
(c) smelting
(d) slagging
Answer
A
Question. The role of calcination in metallurgical operations is
(a) to remove moisture
(b) to decompose carbonates
(c) to drive off organic matter
(d) to decompose carbonates and drive off moisture and organic matter
Answer
D
Question. Van Arkel method of purification of metals involves converting the metal to a
(a) volatile stable compound
(b) volatile unstable compound
(c) non volatile stable compound
(d) None of the above
Answer
A
Question. Process followed before reduction of carbonate ore is
(a) calcination
(b) roasting
(c) liquation
(d) polling
Answer
A
Question. The most abundant metal on the surface of the earth is
(a) Fe
(b) Al
(c) Ca
(d) Na
Answer
B
Question. Calcination is the process in which :
(a) ore is heated above its melting point to expel H2O or CO2 or SO2
(b) ore is heated below its melting point to expel volatile impurities
(c) ore is heated above its melting point to remove S, As and Sb as SO2 ,As2O3 and Sb2O3 respectively
(d) ore is heated below its melting point to expel H2O or CO2
Answer
D
Question. Which of the following statements regarding chromatography is incorrect ?
(a) It is based on the principle that different components of mixture gets adsorbed differently on an adsorbent
(b) Column chromatography involves column of Al2O3 in a glass tube as a stationary phase.
(c) The mobile phase may be a gas, a liquid or a solid.
(d) Component which is more soluble is stationary phase takes longer time to travel.
Answer
C
Question. Which of the following fluxes is used to remove acidic impurities in metallurgical process?
(a) Silica
(b) Lime stone
(c) Sodium chloride
(d) Sodium carbonate
Answer
B
Question. Which of the following metal is leached by cyanide process ?
(a) Ag
(b) Na
(c) Al
(d) Cu
Answer
A
Question. Method used for obtaining highly pure silicon which is used as a semiconductor material, is
(a) oxidation
(b) electrochemical
(c) crystallization
(d) zone refining
Answer
D
Question. Which of the following reactions is an example for calcination process ?
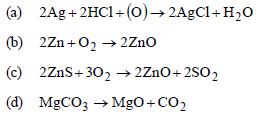
Answer
D
Question. Composition of azurite mineral is
(a) CuCO3CuO
(b) Cu(HCO3)2. Cu(OH)2
(c) 2CuCO3.Cu(OH)2
(d) CuCO3. 2Cu(OH)2
Answer
C
Question. After partial roasting the sulphide of copper is reduced by
(a) cyanide process
(b) electrolysis
(c) reduction with carbon
(d) self reduction
Answer
D
Question. 2CuFeS2 + O2 → Cu2S + 2FeS +SO2
Which process of metallurgy of copper is represented by above equation?
(a) Concentration
(b) Roasting
(c) Reduction
(d) Purification
Answer
B
Question. Cassiterite is concentrated by
(a) levigation
(b) electromagnetic separation
(c) floatation
(d) liquefaction
Answer
B
Question. Which of the following is not used as a collector ?
(a) Pine oil
(b) Xanthates
(c) Cresols
(d) Fatty acids
Answer
C
Question. The main reactions occurring in blast furnace during extraction of iron from haematite are________.
(i) Fe2O3 + 3CO → 2Fe + 3CO2
(ii) FeO + SiO2 → FeSiO3
(iii) Fe2O3 + 3C → 2Fe + 3CO
(iv) CaO + SiO2 → CaSiO3
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i) and (iv)
(d) (i), (ii) and (iii)
Answer
C
STATEMENT TYPE QUESTIONS
Question. Which of the following statements related to Ellingham diagrams are correct ?
(i) It provides a sound basis for the choice of reducing agent in the reduction of oxides.
(ii) Each Ellingham plot is represented by a straight line untill unless there is some change in phase i.e. solid → liquid, liquid → gas and gas → liquid occurs.
(iii) Diagrams similar to Ellingham can be constructed for sulphides and halides which clearly indicates why reduction of MxS is difficult in comparison to MxO.
(iv) Ellingham diagrams predicts the tendency of reduction with a reducing agent and kinetics of the reduction process.
(a) (i), (ii) and (iii)
(b) (i) and (iii)
(c) (i), (ii) and (iv)
(d) (ii) and (iv)
Answer
B
Question. Read the following statements
(i) The principle that the impurities are more soluble in the melt than in the solid state is used in the manufacture of high purity semiconductors.
(ii) Van Arkel method of refining Zr involves heating of crude metal with Cl2 to form corresponding halide.
(iii) Mond process for refining of nickel involves formation of metal carbonyls as an intermediate.Which of the following is the correct code for the statements above ?
(a) TTT
(b) FFT
(c) TFT
(d) FTF
Answer
C
Question. Read the following statements
(i) Magnetic separation method is employed when one component either ore or gangue is magnetic in nature.
(ii) Depressant NaCN used in case of ore containing mixture of ZnS and PbS allows ZnS to come with froth and prevents PbS from coming to the froth.
(iii) For concentration powdered bauxite ore is digested with conc.NaOH at 473–523K and 35–36 bar pressure.Which of the following is the correct code for the statements above ?
(a) TFT
(b) TTF
(c) FTF
(d) FFT
Answer
A
Question. Which of the following statement(s) is/are correct ?
(i) Cast iron is used in the manufacture of railway sleepers
(ii) Wrought iron is used in the manufacture of anchors, bolts, chains etc.
(iii) Nickel steel is used in making pendulums.
(a) Only (i)
(b) (i) and (ii)
(c) (i), (ii) and (iii)
(d) Only (iii)
Answer
C
MATCHING TYPE QUESTIONS
Question. Match the columns.
Column-I Column-II
(A) Coloured bands (p) Zone refining
(B) Impure metal to volatile (q) Fractional distillation
complex
(C) Purification of Ge and Si (r) Mond Process
(D) Purification of mercury (s) Chromatography
(t) Liquation
(a) A – (p), B – (q), C – (s), D – (t)
(b) A – (s), B – (r), C – (p), D – (q)
(c) A – (r), B – (s), C – (p), D – (q)
(d) A – (t), B – (s), C – (r), D – (q)
Answer
B
Question. Match the columns.
Column-I Column-II
(A) Blisterred Cu (p) Aluminium
(B) Blast furnace (q) 2Cu2O + Cu2S → 6Cu + SO2
(C) Reverberatory (r) Iron
furnace
(D) Hall-Heroult (s) FeO + SiO2 → FeSiO3
process
(t) 2Cu2S + 3O2 → 2Cu2O + 2SO2
(a) A – (q), B – (r), C – (s), D – (p)
(b) A – (p), B – (q), C – (r), D – (t)
(c) A – (t), B – (s), C – (r), D – (q)
(d) A – (s), B – (t), C – (r), D – (q)
Answer
A
Question. Match the columns
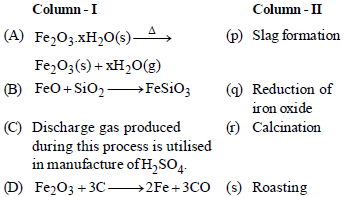
(a) A– (r), B – (p), C – (s), D – (q)
(b) A– (p), B – (r), C – (s), D – (q)
(c) A– (r), B – (s), C – (p), D – (q)
(d) A– (r), B – (p), C – (q), D – (s)
Answer
A
ASSERTION-REASON TYPE QUESTIONS
Directions : Each of these questions contain two statements,Assertion and Reason. Each of these questions also has four alternative choices, only one of which is the correct answer. You have to select one of the codes (a), (b), (c) and (d) given below.
(a) Assertion is correct, reason is correct; reason is a correct explanation for assertion.
(b) Assertion is correct, reason is correct; reason is not a correct explanation for assertion
(c) Assertion is correct, reason is incorrect
(d) Assertion is incorrect, reason is correct.
Question. Assertion : Levigation is used for the separation of oxide ores from impurities.
Reason : Ore particles are removed by washing in a current of water.
Answer
C
Question. Assertion : Zinc can be used while copper cannot be used in the recovery of Ag from the complex [Ag(CN)2]–.
Reason : Zinc is a powerful reducing agent than copper.
Answer
A
Question. Assertion : Leaching is a process of reduction.
Reason : Leaching involves treatment of the ore with a suitable reagent so as to make it soluble while impurities remains insoluble.
Answer
D
Question. Assertion : Coke and flux are used in smelting.
Reason : The phenomenon in which ore is mixed with suitable flux and coke is heated to fusion is known as smelting.
Answer
B
Question.Assertion : Copper obtained after bessemerization is known as blister copper.
Reason : Blisters are produced on the surface of the metal due to escaping of dissolved SO2.
Answer
A
Question. Assertion : Lead, tin and bismuth are purified by liquation method.
Reason : Lead, tin and bismuth have low m.p. as compared to impurities
Answer
A
CRITICAL THINKING TYPE QUESTIONS
Question. Consider the following reactions at 1000°C
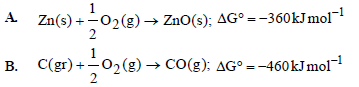
Choose the correct statement at 1000°C
(a) zinc can be oxidised by carbon monoxide.
(b) zinc oxide can be reduced by graphite
(c) carbon monoxide can be reduced by zinc.
(d) both statements (a) and (b) are true
Answer
B
Question. Carbon and CO gas are used to reduce which of the following pairs of metal oxides for extraction of metals ?
(a) FeO, SnO
(b) SnO, ZnO
(c) BaO, Na2O2
(d) FeO, ZnO
Answer
D
Question. The value of ΔG0f for formation of Cr2O3 is – 540 kJmol–1 and that of Al2O3 is – 827 kJ mol–1 What is the value of ΔrG° for the reaction?
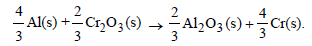
(a) – 574 kJ mol–1
(b) –287 kJ mol–1
(c) + 574 kJ mol–1
(d) +287 kJ mol–1
Answer
B
Question. Which of the following statement is not correct about Ellingham diagram?
(a) ΔG increases with an increase in temperature
(b) It consists of plots of ΔfGº vs T for formation of oxides
(c) a coupling reaction can be well expressed by this diagram
(d) It express the kinetics of the reduction process
Answer
D
Question. In Hall-Heroult process how much carbon anode is burnt away to produce each 1kg of aluminium ?
(a) 0.3 kg
(b) 0.5 kg
(c) 1 kg
(d) 0.1 kg
Answer
B
Question. A coupled reaction takes place as follow–
A + B → C + D, Δ Gº = + x kj
D + E → F Δ Gº = – y kj
for the spontaneity of reaction A + B + E → C+F, which of the following is correct?
(a) 2x = y
(b) x < y
(c) x > y
(d) x = (y)× TΔS
Answer
D
Question. Sulfide ores are common for the metals
(a) Ag, Cu and Pb
(b) Ag, Mg and Pb
(c) Ag, Cu and Sn
(d) Al, Cu and Pb
Answer
A
Question. In the metallurgy of aluminium _________.
(a) Al3+ is oxidised to Al (s).
(b) graphide anode is oxidised to carbon monoxide and carbon dioxide.
(c) oxidation state of oxygen changes in the reaction at anode.
(d) oxidation state of oxygen changes in the overall reaction involved in the process.
Answer
B
Question. Before introducing FeO in blast furnace , it is converted to Fe2O3 by roasting so that
(a) it may not be removed as slag with silica
(b) it may not evaporate in the furnace
(c) presence of it may increase the m.pt. of charge
(d) None of these.
Answer
A
Question. Which of the following metal is correctly matched with its ore?
Metal Ore
(a) Zinc Calamine
(b) Silver Ilmenite
(c) Magnesium Cassiterite
(d) Tin Azurite
Answer
A
Question. Which of the following is incorrectly matched ?
Metal Uses
(a) Wrought iron Casting stoves, gutter pipes, toys etc.
(b) Copper Coinage alloy
(c) Aluminium Extraction of chromium and manganese
(d) Nickel steel Measuring tapes
Answer
A
Question. During the process of electrolytic refining of copper, some metals present as impurity settle as ‘anode mud’. These are
(a) Fe and Ni
(b) Ag and Au
(c) Pb and Zn
(d) Sn and Ag
Answer
B
Question. In the extraction of chlorine from brine_________.
(i) ΔGΘ for the overall reaction is negative.
(ii) ΔGΘ for the overall reaction is positive.
(iii) EΘ for overall reaction has negative value.
(iv) EΘ for overall reaction has positive value.
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) (iii) and (iv)
Answer
B
Question. Germanium of very high purity is obtained by
(a) liquation
(b) vapour phase refining
(c) distillation
(d) zone refining
Answer
D
Question. In electro-refining of metal the impure metal is used to make the anode and a strip of pure metal as the cathode, during the electrolysis of an aqueous solution of a complex metal salt. This method cannot be used for refining of
(a) Silver
(b) Copper
(c) Aluminium
(d) Sodium
Answer
D
Question. Which of the following statements regarding electrolytic refining of copper is incorrect ?
(a) In this process anode is made up of impure copper and pure copper strips are taken as cathode.
(b) Acidic or basic solution of copper sulphate is used as electrolyte
(c) Antimony, tellurium, silver and gold are some of the metals deposits as anode mud during this process
(d) Zinc can be also refined by electrolytic refining method.
Answer
B
Question. Which of the following statements regarding metallurgy of iron is incorrect ?
(a) Reaction Fe3O4 + 4CO → 3Fe + 4CO2 belongs to lower temperature range (500 – 800K) of the blast furnace.
(b) Reaction FeO + CO → Fe + CO2 belongs to higher temperature range (900 – 1500K) of the blast furnace.
(c) The iron obtained from blast furnace is cast iron with 3% carbon.
(d) For reduction of iron oxide to occur ΔG of the couple of following reactions should be negative

Answer
C
Question. If the impurities in a metal has a greater affinity for oxygen and is more easily oxidised than the metal, then the purification of metal may be carried out by
(a) distillation
(b) zone refining
(c) electrolytic refining
(d) cupellation
Answer
D


