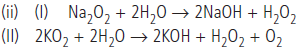Please refer to The s-Block Elements Class 11 Chemistry Important Questions with solutions provided below. These questions and answers have been provided for Class 11 Chemistry based on the latest syllabus and examination guidelines issued by CBSE, NCERT, and KVS. Students should learn these problem solutions as it will help them to gain more marks in examinations. We have provided Important Questions for Class 11 Chemistry for all chapters in your book. These Board exam questions have been designed by expert teachers of Standard 11.
Class 11 Chemistry Important Questions The s-Block Elements
Very Short Answer Type Questions :
Question. Halides of Be dissolve in organic solvents while those of Ba do not. Why is it so?
Answer : This is because halides of Be are covalent while those of Ba are ionic.
Question. BeCl2 in aqueous solution exists as [Be(OH)4]2+. Explain.
Answer : Due to small size and high ionisation enthalpy, Be forms coordination compound.
Question. Explain why Cs is used in photoelectric cell?
Answer : Calcium is used in photoelectric cell due to low value of ionization potential.
Question. Which has a higher melting point, sodiumor potassium?
Answer : Sodium has higher melting point than potassium because of stronger metallic bonding.
Question. What will be formed when lithium nitrate is heated?
Answer : Lithium nitrate on heating decomposes to give Li2O, NO2 and O2
4LiNO3 →Δ 2Li2O + 4NO2 + O2
Question. Potassium carbonate cannot be prepared by Solvay process. Why ?
Answer : Solvay process cannot be extended to the manufacture of K2CO3 because KHCO3 is too soluble to be precipitated by the addition of ammonium hydrogen carbonate to a saturated solution of potassium chloride.
Question. Why are group 2 elements harder than group 1 elements?
Answer : This is because the smaller atomic size causes the electrons to be packed more closely, thereby forming strong metallic bonds.
Question. Why are lithium salts commonly hydrated and those of the other alkali ions are usually anhydrous?
Answer : Because of smallest size among alkali metals, Li+ can polarise water molecules more easily than the other alkali metal ions and hence get attached to lithium salts as water of crystallisation. For example, lithium chloride crystallises as LiCl.2H2O but sodium chloride as NaCl.
Question. Find out the oxidation state of sodium in Na2O2.
Answer : Let oxidation state of Na = x
The oxidation state of O = –1 (present as peroxide)
∴ 2x + 2(–1) = 0 ⇒ 2x – 2 = 0
⇒ 2x = 2 ⇒ x = +1
∴ Oxidation state of Na in Na2O2 is +1.
Question. Why are the first ionization enthalpies of group 1 elements low?
Answer : The ionization enthalpy, is the energy required to completely remove an electron from an isolated gaseous atom or ion. The closer and more tightly an electron is bound to its nucleus, the more difficult it will be to remove the electron and higher will be the ionization enthalpy. Group 1 elements have low first ionization enthalpies because the loss of an electron takes place readily to form a stable octet.
Short Answer Type Questions :
Question. What happens when :
(i) sodium peroxide dissolved in hot water?
(ii) sodium metal is heated in free supply of air?
Answer : (i) Sodium hydroxide and hydrogen peroxide will be formed.

Question. (a) Write balanced equations for the reactions:
(i) alkali metal and water
(ii) alkali metal and dihydrogen.
(b) The E° for Cl2/Cl– is +1.36, for I2/I– is + 0.53, for Ag+/Ag is +0.79, for Na+/Na is – 2.71 and for Li+/Li is – 3.04. Arrange the following ionic species in decreasing order of reducing strength:
I–, Ag, Cl–, Li, Na
Answer :
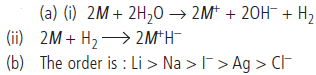
Question. Given reason:
(i) Alkali metals impart colours to Bunsen Burner flame.
(ii) KO2 is paramagnetic.
(iii) Alkaline earth metals give-blue solutions, when dissolved in liquid ammonia.
Answer : (i) Alkali metals and their salts impart colour to flame. It is because their loosely held valence electrons get excited to higher energy level. When they return back they release visible light of characteristic colour to flame.
(ii) In KO2, superoxide ion O2– is present. O2– ion has one unpaired electron in its antibonding molecular orbital. Hence, it is paramagnetic in nature.
(iii) Like alkali metals the alkaline earth metals dissolve in liquid ammonia to give deep blue black solution forming ammoniated ions.
M + (x + y)NH3 → [M(NH3)x]2+ + 2[e(NH3)y]–
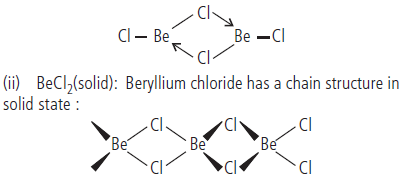
Question. Draw the structure of (i) BeCl2 (vapour) (ii) BeCl2 (solid).
Answer : (i) BeCl2 exists as a dimer in vapour phase, which dissolves into the linear monomer Cl–Be–Cl at 1200 K.
Question. Discuss the general characteristics and gradation in properties of alkaline earth metals.
Answer : (i) Electronic configuration : The valence electronic configuration of atoms of the group II A elements is ns2, where ‘n’ is the period number.
(ii) Atomic and ionic sizes : The size of the atom increases gradually from Be to Ra. Their ions are also large and size of the ion increases from Be2+ to Ra2+.
(iii) Ionisation enthalpy : The 1st and 2nd ionisation energies of these metals decrease from Be to Ba as size increases.
Question. Compare the alkali metals and alkaline earth metals with respect to
(i) ionisation enthalpy
(ii) basicity of oxides and
(iii) solubility of hydroxides.
Answer : (i) Ionisation Enthalpy : The ionisation energies of alkaline earth elements are higher than those of alkali metals due to higher nuclear charge and smaller radii.
(ii) Basicity of oxides : Oxides of alkali metals are stronger bases as compared to those of alkaline earth metals present in the same period. e.g., when Na2O is dissolved in water, NaOH formed is a stronger base than when MgO is dissolved in water to form Mg(OH)2.This is due to higher ionisation
energies of alkaline earth metals.
(iii) Solubility of hydroxides : Alkali metal hydroxides are more soluble in water as compared to the hydroxides of alkaline earth metals present in the same period. This is due to higher lattice energy of the hydroxides of alkaline earth elements as compared to those of alkali metals.
Question. What happens when :
(a) sodium metal is dropped in water?
(b) sodium metal is dissolved in liquid ammonia?
Answer : (a) Sodium hydroxide and hydrogen gas will be formed which will catch fire.
2Na(s) + 2H2O(l) → 2NaOH(aq) + H2(g)
(b) Sodium metal dissolves in liquid ammonia and gives deep blue solution which is conducting in nature.
Na + (x + y) NH3 → [Na(NH3)x]+ + [e(NH3)y]–
The blue colour of the solution is due to the ammoniated electron which absorbs energy in the visible region of light and thus imparts blue colour to the solution.
Question. Give reason:
(a) Sodium is less reactive than potassium.
(b) Be and Mg do not give colour to the flame whereas other alkaline earth metals do so.
(c) Sodium is stored under kerosene oil.
Answer : (a) This is mainly due to high ionisation enthalpy of sodium as compared to potassium. Therefore, potassium is more electropositive, reactive and a stronger reducing agent than sodium.
(b) In Be and Mg, the electrons are tightly held and excitation by mere flame is rather difficult, thus, they do not show flame colouration.
(c) Sodium is stored in kerosene oil because in air, sodium is easily oxidised to oxide which may dissolve in the moisture to form hydroxide.
Question. (i) Name the chief factors responsible for the anomalous behaviour of lithium.
(ii) Complete the following reactions :
(a) 4LiNO3 →Δ
(b) 2NaNO3 →Δ
Answer : (i) Chief factors responsible for the anomalous behaviour of lithium are :
(I) its very small size
(II) high electronegativity
(III) high ionization enthalpy and
(IV) absence of vacant d-atomic orbital in the valence shell.
(ii) (a) 4LiNO3 →Δ 2Li2O + 4NO2 + O2
(b) 2NaNO3 →Δ 2NaNO2 + O2
Question. Explain term Diagonal Relationship in context of Be and Al. Give two points of similarities between them.
Answer : The charge/radius ratio of Be2+ is nearly the same as that of Al3+ ion. Hence, beryllium resembles aluminium. This is known as diagonal relationship.
(i) Like aluminium, beryllium is not readily attacked by acids because of the presence of an oxide film on the surface of the metal.
(ii) Beryllium hydroxide dissolves in excess of alkali to give a beryllate ion, [Be(OH)4]2– just as aluminium hydroxide gives aluminate ion, [Al(OH)4]–.
Question. Alkaline earth metals always form divalent cations even though their second ionization enthalpies are almost double than their first ionization enthalpies. Explain.
Answer : In the solid state, higher enthalpy of lattice formation by M2+ ions (as compared to M+ ions) more than compensates the higher second ionization enthalpies of metals and in aqueous solution, higher enthalpy of hydration of M2+ ions (as compared to M+ ions) more than compensate at the higher second ionization enthalpy of metals.
Question. How would you explain the following observations?
(i) BeO is almost insoluble but BeSO4 is soluble in water,
(ii) LiI is more soluble than KI in ethanol.
Answer : (i) BeO is almost insoluble in water as it is covalent in nature and tightly held together in the solid state while BeSO4 is highly soluble in water due to high energy of solvation of smaller Be2+ ion.
(ii) LiI having much more covalent character than KI because of small size of Li+ as compared to K+. Hence, LiI is more soluble in ethanol.
Question. (i) Why are alkali metal used in photoelectric cells?
(ii) What happens when K burns in air? Write equation.
Answer : (i) Alkali metals are used in photoelectric cells and because of their low ionization energies they lose electrons very easily on irradiation.
(ii) Potassium reacts with oxygen to form superoxide.
K + O2 → KO2
Question. An element of group 2 forms covalent oxide which is amphoteric in nature and dissolves in water to give an amphoteric hydroxide. Identify the element and write chemical reactions of the hydroxide of the element with an alkali and an acid.
Answer : The element is beryllium.

Question. Comment on the following observations :
(a) Why are alkali metals not found in nature?
(b) The mobilities of the alkali metal ions in aqueous solution are Li+ < Na+ < K+ < Rb+ < Cs+
Answer : (a) All the alkali metals have one valence electron, ns1, outside the noble gas core. The loosely held s-electron in the outermost valence shell of these elements makes them the most electropositive metals. They readily lose an electron to give monovalent M+ ions. Thus, due to the reason cited above, alkali metals are never found free in nature but are always found in combined state.
(b) This is attributed to the hydration of the cation in water. As a result, size of the cation increases and its mobility decreases. Due to the smallest size, Li+ ion is hydrated to the maximum and has least mobility while Cs+ ion due to least hydration has maximum mobility.
Question. Account for the following:
(i) Magnesium does not show any flame colouration.
(ii) Group 1 elements have low melting and boiling points.
Answer : (i) In Mg, the electrons are tightly held and excitation by mere flame is rather difficult, thus, Mg does not show flame colouration.
(ii) As group 1 elements have large size, the intermetallic bonds in them are quite weak. Hence they have low melting and boiling points.
Question. Why is Li2CO3 decomposed at a lower temperature whereas Na2CO3 at higher temperature?
Answer : Li2CO3 decomposes on heating because the Li+ ion exerts a strong polarising action and distorts the electron cloud of the nearby oxygen atom of the large CO32– ion. This results in the weakening of the C—O bond and strengthening of the Li—O bond. This ultimately facilitates the decomposition of Li2CO3 into Li2O and CO2. The lattice energy of Li2O is higher than the lattice energy of Li2CO3. This also favours decomposition of Li2CO3. Na+ ion being bigger in size, the lattice energy of Na2O is less stable than that of Na2CO3. Therefore, Na2CO3 does not decompose on heating.
Question. Give reason :
(i) Alkali metals are strong reducing agents
(ii) Alkali metals are soft metals
(iii) Alkali metals tarnish in air easily.
Answer : (i) The alkali metals have only one electron in their valence shell which they lose easily, owing to their low ionization enthalpies, hence they are strong reducing agents.
(ii) Due to the presence of weak metallic bonding, alkali metals are soft and can be cut with a knife.
(iii) Lithium, sodium and potassium are all soft metals that are easily cut with a knife. The freshly cut surface is a shiny, silver colour, but this tarnishes quickly to a dull grey as the metal reacts with oxygen and water present in the air.
Question. Complete the reactions :
(a) Be(OH)2 + 2OH–
(b) BaO + H2O
Answer :
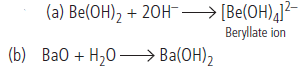
Question. Compare the solubility and thermal stability of the following compounds of the alkali metals with those of the alkaline earth metals.
(a) Nitrates
(b) Carbonates
(c) Sulphates
Answer : (a) Nitrates : Nitrates of alkali metals are of the type MNO3, they are soluble in water and do not undergo hydrolysis. Except LiNO3, other nitrates of this group decompose to nitrites and oxygen upon heating.
2MNO3 → 2MNO2 + O2↑
Nitrates of alkaline earth metals are of the type M(NO3)2. They are soluble in water and upon heating they decompose into their corresponding oxides with evolution of a mixture of NO2 and oxygen.
2M(NO3)2 →Δ 2MO + 4NO2 + O2↑
(b) Carbonates : All alkali metals form carbonates of the type M2CO3. Their carbonates are highly stable towards heat and readily soluble in water. The stability and solubility of the carbonates increases in the same order:
Cs2CO3 > Rb2CO3 > K2CO3 > Na2CO3 > Li2CO3.
Li2CO3 decomposes on heating and is insoluble in water. Carbonates of alkaline earth metals (MCO3) are insoluble in water, they dissolve in water only in presence of CO2 due to the formation of a hydrogen carbonate.
MCO3 + H2O + CO2 M(HCO3)2
Solubility of carbonates decreases as we descend the group and stability increases due to increase in electropositive character of the metal.
(c) Sulphates : Alkali metal sulphates are of the type M2SO4. Except Li2SO4, all other sulphates are soluble in water. Alkaline earth metal sulphates are of the type MSO4. The solubility of sulphates decreases on moving down the group. CaSO4 is sparingly soluble, SrSO4, BaSO4 and RaSO4 are almost insoluble. The solubilities of BeSO4 and MgSO4 are due to high energy of solvation of smaller Be2+ and Mg2+ ions. The order of solubility is :
BaSO4 < SrSO4< CaSO4< MgSO4 < BeSO4.
The sulphates decompose on heating to give the corresponding oxide (MO).
2MSO4 →Δ 2MO + 2SO2 + O2
The stability increases as the basic nature of the metal increases.
SrSO4 > CaSO4 > MgSO4> BeSO4.
Question. (a) Among the given alkali metals, which has least melting point? Na, K, Rb, Cs
(b) Which one of the following alkali metals gives hydrated salts? Li, Na, K, Cs
Answer : (a) Atomic size increases as we move down the group. As a result, the strength of metallic bonding decreases on moving down a group in the periodic table. This causes a decrease in the melting point. Among the given metals, the size of Cs is the largest and thus it has the least melting point.
(b) Smaller the size of an ion, the more highly it is hydrated. Among alkali metal ions, Li+ ion is smallest in size. Also , it has the highest charge density and highest polarizing power. Hence, it attracts water molecules more strongly than the other alkali metal ions. As a result, it forms hydrated salts such as LiCl·2H2O. The other alkali metal ions are larger in size than Li+ and have weaker charge densities. Hence, they usually do not form hydrated salts.
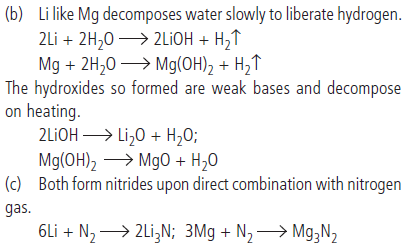
Question. In what ways lithium shows similarities to magnesium in its chemical behaviour?
Answer : (a) Both Li and Mg are harder and lighter than the other metals in their respective groups.
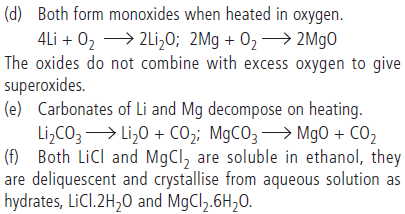
Question. Complete the reactions:
(i) Na + O2
(ii) BeCl2 + LiAIH4
(iii) NaNO3 →Δ
Answer :
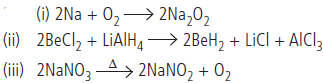
Question. On combustion Li forms Li2O; sodium gives the peroxide, Na2O2; and potassium, rubidium and caesium give superoxides, MO2. Why Li does not form a peroxide?
Answer :
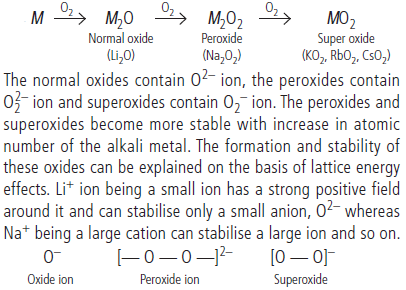
Question. Arrange the following in order of property mentioned against each :
(i) BeCl2, MgCl2, CaCl2, BaCl2 [Increasing ionic character]
(ii) Mg(OH)2, Ca(OH)2, Ba(OH)2, Sr(OH)2 [Increasing solubility in water]
Answer : (i) BeCl2 < MgCl2 < CaCl2 < BaCl2
(ii) Mg(OH)2 < Ca(OH)2 < Sr(OH)2 < Ba(OH)2
Long Answer Type Questions :
Question. (a) Explain why can alkali and alkaline earth metals not be obtained by chemical reduction methods.
(b) Explain the following :
(i) Be and Mg do not impart any colour to the flame.
(ii) What happens when Mg is burnt in air?
(c) Account for the following:
(i) Magnesium does not show any flame colouration.
(ii) Group 1 elements have low melting and boiling points.
Answer : (a) Alkali metals cannot be extracted by the reduction of their oxides and other compounds as they are strong reducing agents themselves and no such reducing agents are there which can reduce them to get pure metal.
(b) (i) In Be and Mg, the electrons are tightly held and excitation by mere flame is rather difficult, thus, they do not show flame colouration.
(ii) Mg burns in air to form MgO and Mg3N2.
2Mg + O2 → 2MgO
3Mg + N2 → Mg3N2
(c) (i) In Mg, the electrons are tightly held and excitation by mere flame is rather difficult, thus, Mg does not show flame colouration.
(ii) As group 1 elements have large size, the intermetallic bonds in them are quite weak. Hence they have low melting and boiling points.
Question. (a) When a metal of group 1 was dissolved in liquid ammonia, the following observations were obtained:
(i) Blue solution was obtained initially.
(ii) On concentrating the solution, blue colour changed to bronze colour.
How do you account for the blue colour of the solution? Give the name of the product formed on keeping the solution for some time.
(b) The stability of peroxide and superoxide of alkali metals increase as we go down the group. Explain giving reason.
Answer : (a) (i) Alkali metals dissolve in liquid ammonia giving deep blue solutions which are conducting in nature.
M+ (x + y)NH3 [M(NH3)x]+ + [e(NH3)y]–
Blue colour is due to ammoniated electron.
(ii) On standing, hydrogen is slowly liberated and amide is formed. The colour changes to bronze. On keeping for sometime.
M+ + e– + NH3 MNH2 +1/2 H2
(b) The increase in stability of peroxides and superoxides of alkali metals from Li to Cs can be explained by stabilisation of larger anions by larger cations through higher lattice energy.
Question. Present a comparative account of the alkali and alkaline earth metals with respect to the following characteristics :
(i) Tendency to form ionic/ covalent compounds
(ii) Nature of oxides and their solubility in water
(iii) Formation of oxosalts
(iv) Solubility of oxosalts
(v) Thermal stability of oxosalts
Answer : (i) Tendency to form ionic/covalent compounds :
(a) All common compounds of alkali metals are generally ionic in nature. Halides and oxides are ionic in nature with exception of lithium compounds which are generally covalent in nature.
(b) Alkaline earth metals form ionic oxides and halides except Be which forms covalent compounds.
(ii) Nature of oxides and their solubility in water :
(a) Alkali metals form oxides, peroxides and superoxides. These oxides are basic in nature and basic character increases down the group. Oxides dissolve in water to give hydroxides. These hydroxides are strong bases.
(b) Alkaline earth metal oxides are basic in nature but less basic than alkali metal oxides. BeO is amphoteric while other oxides are basic and form sparingly soluble hydroxides.
(iii) Formation of oxosalts :
(a) Alkali metals form sulphates, carbonates and bicarbonates.
(b) Alkaline earth metals form sulphates, carbonates and nitrates.
(iv) Solubility of oxosalts :
(a) Alkali metal oxosalts are generally soluble in water. Solubility increases down the group.
(b) Carbonates and sulphates of alkaline earth metals become insoluble as we move down the group.
(v) Thermal stability of oxosalts :
(a) Alkali metal oxosalts are thermally stable and stability increases down the group. Li2CO3 and Li2SO4 decompose on heating.
(b) Alkaline earth metals carbonates decompose on heating while their thermal stability increases down the group.
Question. The s-block elements are characterised by their larger atomic sizes, lower ionisation enthalpies, invariable +1 oxidation state and solubilities of their oxosalts. In the light of these features describe the nature of their oxides, halides and oxosalts.
Answer : (i) Nature of oxides – Alkali metals form M2O, M2O2 and MO2 types of oxides. The stability of the peroxide or superoxide increases as the size of metal cation increases, this is due to stabilisation of large anions by larger cations.
(ii) Nature of halides – Alkali metal halides have general formula MX. All halides are soluble in water. LiF is very less soluble in water due to its high lattice energy. Their m.pt. and b.pt. follow the trend – fluoride > chloride > bromide > iodide due to increase in size of halide ion, the lattice energy increases.
(iii) Oxosalts – Oxosalts of alkali metals are generally soluble in water and thermally stable. As electropositive character increases down the group, stability of carbonates and bicarbonates increases.
Question. (a) (i) What are the products formed when alkali metal oxide (M2O), peroxide (M2O2) and superoxide (MO2) hydrolysed by water?
(ii) Why sodium forms peroxide but potassium forms superoxide?
(b) (i) Can we store sodium in water or not? Why.
(ii) Write balanced equations for the reactions between :
(I) Na2O2 and H2O
(II) KO2 and H2O.
Answer : (a) (i) Alkali metal oxide, peroxide and superoxide are easily hydrolysed by water to form the hydroxides according to the following reactions:
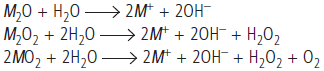
(ii) Sodium ion is larger in size and has comparatively weaker +ve field around it which can not prevent oxide ion to combine with another oxygen atom to form peroxide ion. While K+ is larger than Na+ and has still weaker +ve field which can not prevent even peroxide ion to combine with another oxygen atom to form superoxide.
(b) (i) Sodium will react with water to form sodium hydroxide and hydrogen gas which will catch fire. For this reason, sodium cannot be stored in water.