VBQs The d – and f – Block Elements Class 12 Chemistry with The d – and f – Block Elements has been provided below for standard students. We have provided chapter wise VBQ for Class 12 Chemistry with The d – and f – Block Elements. The following The d – and f – Block Elements Class 12 Chemistry value based questions with answers will come in your exams. Students should understand the concepts and learn the solved cased based VBQs provided below. This will help you to get better marks in class 12 examinations.
The d – and f – Block Elements VBQs Class 12 Chemistry
Question. Which of the following actinoids show oxiation states upto +7 ?
(i) Am
(ii) Pu
(iii) U
(iv) Np
(a) (i) and (ii)
(b) (ii) and (iv)
(c) (iii) and (iv)
(d) (i) and (iii)
Answer
B
Question. Which of the following exhibit only + 3 oxidation state ?
(a) U
(b) Th
(c) Ac
(d) Pa
Answer
C
Question. Total number of inner transition elements in the periodic table is
(a) 10
(b) 14
(c) 28
(d) 30
Answer
C
Question. The lanthanoide contraction is responsible for the fact that
(a) Zr and Y have about the same radius
(b) Zr and Nb have similar oxidation state
(c) Zr and Hf have about the same radius
(d) Zr and Zn have the same oxidation state
Answer
C
Question. Which one of the following elements shows maximum number of different oxidation states in its compounds?
(a) Eu
(b) La
(c) Gd
(d) Am
Answer
D
Question. Lanthanoids are
(a) 14 elements in the sixth period (atomic no. = 90 to 103) that are filling 4f sublevel
(b) 14 elements in the seventh period (atomic no. = 90 to 103) that are filling 5f sublevel
(c) 14 elements in the sixth period (atomic no. = 58 to 71) that are filling 4f sublevel
(d) 14 elements in the seventh period (atomic no. = 58 to 71) that are filling 4f sublevel
Answer
C
Question. In which of the following lanthanides oxidation state +2 is most stable?
(a) Ce
(b) Eu
(c) Tb
(d) Dy
Answer
B
Question. Lanthanoid which has the smallest size in +3 state is
(a) Tb
(b) Er
(c) Ce
(d) Lu
Answer
D
Question. Lanthanum is grouped with f-block elements because
(a) it has partially filled f-orbitals
(b) it is just before Ce in the periodic table
(c) it has both partially filled f and d-orbitals
(d) properties of lanthanum are very similar to the elements of f-block
Answer
D
Question. A reduction in atomic size with increase in atomic number is a characteristic of elements of
(a) high atomic masses
(b) d-block
(c) f-block
(d) radioactive series
Answer
C
Question. Which of the following actinoid element has 5f 7 6d1 7s2 configuration?
(a) Bk
(b) Cm
(c) Pa
(d) No
Answer
B
Question. Which of the following factors may be regarded as the main cause of lanthanoide contraction?
(a) Greater shielding of 5d electrons by 4f electrons
(b) Poorer shielding of 5d electrons by 4f electrons
(c) Effective shielding of one of 4f electrons by another in the subshell
(d) Poor shielding of one of 4f electron by another in the subshell
Answer
B
Question. Which of the following ions will exhibit colour in aqueous solutions?
(a) La3+ (Z = 57)
(b) Ti3+ (Z = 22)
(c) Lu3+ (Z = 71)
(d) Sc3+ (Z = 21)
Answer
B
Question.In context of the lanthanoids, which of the following statements is not correct?
(a) There is a gradual decrease in the radii of the members with increasing atomic number in the series.
(b) All the members exhibit +3 oxidation state.
(c) Because of similar properties the separation of lanthanoids is not easy.
(d) Availability of 4f electrons results in the formation of compounds in +4 state for all the members of the series.
Answer
D
Question. Which of the following in its oxidation state shows the paramagnetism ?
(a) Tb(IV)
(b) Lu(III)
(c) Ce(IV)
(d) La(III)
Answer
A
Question. The correct order of ionic radii of Y3+, La3+, Eu3+ and Lu3+ is
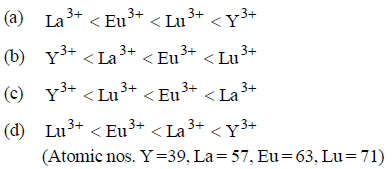
Answer
C
Question. The increasing order of the shielding of electrons by the orbitals ns,np,nd,nf is
(a) ns,np,nd,nf
(b) np,ns,nd,nf
(c) nd,nf,np,ns
(d) nf,nd.np,ns
Answer
D
Question. Which of the following lanthanoid ions is diamagnetic ?
(At nos. Ce = 58, Sm = 62, Eu = 63, Yb = 70)
(a) Sm2+
(b) Eu2+
(c) Yb2+
(d) Ce2+
Answer
C
Question. Which of the following oxidation states is the most common among the lanthanoids?
(a) 3
(b) 4
(c) 2
(d) 5
Answer
A
Question. The approximate percentage of iron in mischmetal is
(a) 10
(b) 20
(c) 50
(d) 5
Answer
D
Question. What is theis the percentage of lanthanoid metal in mischmetall?
(a) 90%
(b) 20%
(c) 5%
(d) 95%
Answer
D
Question. Non-lanthanide atom is
(a) La
(b) Lu
(c) Pr
(d) Pm
Answer
A
Question. Identify the incorrect statement among the following:
(a) 4f and 5f orbitals are equally shielded.
(b) d-Block elements show irregular and erratic chemical properties among themselves.
(c) La and Lu have partially filled d-orbitals and no other partially filled orbitals.
(d) The chemistry of various lanthanoids is very similar.
Answer
A
Question. Actinoides
(a) are all synthetic elements
(b) include element 104
(c) have any short lived isotopes
(d) have variable valency
Answer
D
Question. The outer electronic configuration of Gd (Atomic No. : 64) is
(a) 4f 3 5d5 6s2
(b) 4f 8 5d 0 6s2
(c) 4f 4 5d4 6s2
(d) 4f 7 5d 1 6s2
Answer
D
Question. Larger number of oxidation states are exhibited by the actinoids than those by the lanthanoids, the main reason being
(a) 4f orbitals more diffused than the 5f orbitals
(b) lesser energy difference between 5f and 6d than between 4f and 5d orbitals
(c) more energy difference between 5f and 6d than between 4f and 5d orbitals
(d) more reactive nature of the actionids than the lanthanoids
Answer
B
Question. Lanthanide contraction can be observed in
(a) At
(b) Gd
(c) Ac
(d) Lw
Answer
B
Question. There are 14 elements in actinoid series. Which of the following elements does not belong to this series ?
(a) U
(b) Np
(c) Tm
(d) Fm
Answer
C
Question. Which of the following is not correctly matched?
Compound of Use
transition metal
(a) TiO Pigment industry
(b) MnO2 Dry battery cell
(c) V2O5 Manufacture of H2SO4
(d) PdCl2 Manufacture of polyethylene
Answer
D
Question. Which of the following lanthanoid element is steel hard in nature?
(a) Eu
(b) Pm
(c) Sm
(d) Ce
Answer
C
Question. The most common lanthanide is
(a) lanthanum
(b) cerium
(c) samarium
(d) plutonium
Answer
B
Question. A series1 metal ion , M(II) aqueous solution react with the KI to form iodine and a precipitate is formed, this M(II) can be:
(a) Zn2+
(b) Mn2+
(c) Cu2+
(d) Ni2+
Answer
C
Question. Which of the following is the use of mischmetall ?
(a) In bullets
(b) In lighter flint
(c) As catalyst in petroleum cracking
(d) Both (a) and (b)
Answer
D
Question. The maximum oxidation state exhibited by actinide ions is
(a) +5
(b) +4
(c) +7
(d) +8
Answer
C
STATEMENT TYPE QUESTIONS
Question. Read the following statements?
(i) Aqueous solutions formed by all ions of Ti are colourless.
(ii) Aqueous solution of ferrous ions is green in colour.
(iii) Small size and presence of vacant d-orbitals make transition metal ions suitable for formation of complex compounds.
(iv) Catalytic action of transition metals involves the increase of reactant concentration at catalyst surface and weakening of the bonds in the reacting molecules.
Which of the following is the correct code for above statements?
(a) FTTT
(b) TFFT
(c) TFTT
(d) FFTT
Answer
A
Question. Which of the following statements are correct ?
(i) Chromium has the highest melting point among the series 1 metals.
(ii) Number of unpaired electrons is greater in Cr than other elements of series 1.
(iii) In any row the melting point of transition metal increases as the atomic number increases.
(a) (i) and (iii)
(b) (i) and (ii)
(c) (ii) and (iii)
(d) (i), (ii) and (iii)
Answer
B
Question. Which of the following statement(s) regarding Hf and Zr is/are correct ?
(i) Hf has greater density than Zr.
(ii) Lanthanoid contraction is responsible for such radii.
(a) Both (i) and (ii) are correct.
(b) Both (i) and (ii) are incorrect
(c) Statement (i) is correct only
(d) Statement (ii) is correct only.
Answer
A
Question. Which of the following statements are correct?
(i) Interstitial compounds contain non-metal atoms trapped inside the metal crystal whereas alloys are homogeneous blend of metals.
(ii) Steel and bronze are alloys of transition and nontransition metals.
(iii) Some boride containing interstitial compounds are very hard comparable to that of diamond.
(iv) Interstitial compounds are chemically more reactive than parent metal.
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (ii) and (iii)
(d) (i), (ii) and (iii)
Answer
A
Question. Which of the following statements are correct?
(i) As a result of lanthanoid contraction members of 4d and 5d series exhibit similar radii.
(ii) IE2 is high for Cr and Cu whereas IE3 is very high for Zn.
(iii) Heavier members of d-block elements like p-block elements favours lower oxidation states.
(iv) In any transition series maximum number of oxidation states is shown by middle elements or elements near middle elements.
(a) (i) and (ii)
(b) (i), (ii) and (iv)
(c) (i), (ii) and (iii)
(d) (ii) and (iv)
Answer
B
Question. Mark the correct statement(s).
(i) Manganese exhibits +7 oxidation state
(ii) Zinc forms coloured ions
(iii) [CoF6]3– is diamagnetic
(iv) Sc forms +4 oxidation state
(v) Zn exhibits only +2 oxidation state
(a) (i) and (ii)
(b) (i) and (v)
(c) (ii) and (iv)
(d) (iii) and (iv)
Answer
B
Question. Read the following statements.
(i) Chemistry of actinoids is complex in comparsion to chemistry of lanthanoids.
(ii) Ce4+ is very good reducing agent.
(iii) Eu2+ is a strong reducing agent.
(iv) Out of all lanthanides Ce,Pr,Nd,Dy and Ho shows +4 oxidation state.
Which of the following is the correct code for the statements above?
(a) TTFF
(b) TFTF
(c) FTFT
(d) FTTF
Answer
B
Question. Which of the following statements are correct ?
(i) The maximum oxidation state of Mn with the oxygen is +VII while with fluorine is +IV.
(ii) Fluorine is more oxidizing in nature than oxygen.
(iii) Fluorine exhibit an oxidation state of –1.n
(iv) Seven fluorine cannot be accommodated around M.
(a) (i), (ii) and (iii)
(b) (ii), (iii) and (iv)
(c) (i) and (iv)
(d) (i), (ii), (iii) and (iv)
Answer
D
Question. Read the following statements?
(i) Only Pu show maximum oxidation state of +7 in actinoids.
(ii) M4+ ion of Th is the only diamagnetic M4+ ion of actinoid series.
(iii) Electrons present in the 5f orbitals of actinides can participate in bonding to a firm greater extent as compared to electrons present in 4f orbitals of lanthanides.
(iv) Magnetic properties of actinoids are more complex than lanthanoids Which of the following is the correct code for the statements above?
(a) FTTT
(b) TFTT
(c) TFFT
(d) FFTT
Answer
A
Question. Consider the following statements
(i) La(OH)3 is the least basic among hydroxides of lanthanides.
(ii) Zr4+ and Hf4+ posses almost the same ionic radii.
(iii) Ce4+ can as an oxidizing agent.
Which of the above is/are true ?
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (ii) only
(d) (i) and (ii)
Answer
B