Please refer to MCQ Questions Chapter 1 Chemical Reactions and Equations Class 10 Science with answers provided below. These multiple-choice questions have been developed based on the latest NCERT book for class 10 Science issued for the current academic year. We have provided MCQ Questions for Class 10 Science for all chapters on our website. Students should learn the objective based questions for Chapter 1 Chemical Reactions and Equations in Class 10 Science provided below to get more marks in exams.
Chapter 1 Chemical Reactions and Equations MCQ Questions
Please refer to the following Chapter 1 Chemical Reactions and Equations MCQ Questions Class 10 Science with solutions for all important topics in the chapter.
MCQ Questions Answers for Chapter 1 Chemical Reactions and Equations Class 10 Science
Question. When Ag is exposed to air it gets a black coating of
(a) AgNO3
(b) Ag2S
(c) Ag2O
(d) Ag2CO3
Answer
B
Question. Electrolysis of water is a decomposition reaction. The mole ratio of hydrogen and oxygen gases liberated during electrolysis of water is:
(a) 1 : 1
(b) 2 : 1
(c) 4 : 1
(d) 1 : 2
Answer
B
Question. What type of chemical reactions take place when electricity is passed through water?
(a) Displacement
(b) Combination
(c) Decomposition
(d) Double displacement
Answer
C
Question. Select the oxidising agent for the following reaction:
H2S + I2 → 2HI + S
(a) I2
(b) H2S
(c) HI
(d) S
Answer
A
Question. Which of the following is an endothermic process?
(a) Dilution of sulphuric acid
(b) Sublimation of dry ice
(c) Condensation of water vapours
(d) Respiration in human beings
Answer
B
Question. A substance added to food containing fats and oils is called:
(a) Oxidant
(b) Rancid
(c) Coolant
(d) Antioxidant
Answer
D
Question. The condition produced by aerial oxidation of fats and oils in foods marked by unpleasant smell and taste is called:
(a) antioxidation
(b) reduction
(c) rancidity
(d) corrosion
Answer
C
Question. In the double displacement reaction between aqueous potassium iodide and aqueous lead nitrate, a yellow precipitate of lead iodide is formed. While performing the activity if lead nitrate is not available, which of the following can be used in place of lead nitrate?
(a) Lead sulphate (insoluble)
(b) Lead acetate
(c) Ammonium nitrate
(d) Potassium sulphate
Answer
D
Question. When SO2 gas is passed through saturated solution of H2S, which of the following reaction occurs?
(a) SO2 + 2H2S → 2H2O + 3S
(b) SO2 + 2H2S → H2O + 3S
(c) SO2 + H2S → H2O + S
(d) SO2 + H2O → SO3 + H2
Answer
C
11. A substance ‘X’ is used in white-washing and is obtained by heating limestone in the absence of air. Identify ‘X’.
(a) CaOCl2
(b) Ca (OH)2
(c) CaO
(d) CaCO3
Answer
A
Question. Combination of phosphorus and oxygen is an example of–
(a) oxidation
(b) reduction
(c) rancidity
(d) None of these
Answer
A
Question. A dilute solution of sodium carbonate was added to two test tubes (A) containing dil HCl and (B) containing dilute NaOH. The correct observation was –
(a) a brown coloured gas liberated in test tube A.
(b) a brown coloured gas liberated in test tube B.
(c) a colourless gas liberated in test tube A.
(d) a colourless gas liberated in test tube B.
Answer
C
Question. Hydrogen sulphide (H2S) is a strong reducing agent. Which of the following reactions shows its reducing action?
(a) Cd(NO3)2 + H2S → CdS + + 2HNO3
(b) CuSO4 + H2S → CuS + H2SO4
(c) 2FeCl3 + H2S → 2FeCl2 + 2HCl + S
(d) Pb(NO3)2 + H2S → PbS + 2CH3COOH
Answer
C
Question. Rusting of iron is an example of –
(a) reduction
(b) redox
(c) oxidation
(d) dissociation
Answer
B
Question. Which of the following does not corrode when exposed to the atmosphere?
(a) Iron
(b) Copper
(c) Gold
(d) Silver
Answer
C
Question. In the following equations :
Na2CO3 + x HCl → 2 NaCl + CO2 + H2O, the value of x is–
(a) 1
(b) 2
(c) 3
(d) 4
Answer
B
Question. A balanced chemical equation is in accordance with –
(a) Avogadro’s law
(b) law of multiple proportion
(c) law of conservation of mass
(d) law of gaseous volumes.
Answer
C
Question. In the equation, NaOH + HNO3 → NaNO3 + H2O nitric acid is acting as –
(a) an oxidising agent
(b) an acid
(c) a nitrating agent
(d) a dehydrating agent
Answer
B
Question.
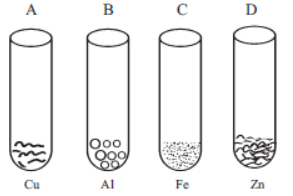
If we added FeSO4 to above four test tubes, in which test tube we observe black residue?
(a) “A” and “B”
(b) “B” and “C”
(c) “A” and “C”
(d) “B” and “D”
Answer
D
Question. Take about 1.0g CaCO3 in a test tube. Heat it over a flame, a colourless gas comes out. The reaction is called a
(a) decomposition reaction
(b) displacement reaction
(c) double decomposition reaction
(d) double displacement reaction
Answer
A
Question. 2CuI → Cu + CuI2, the reaction is –
(a) redox
(b) neutralisation
(c) oxidation
(d) reduction
Answer
A
Question. Identify the endothermic process from the following
(a) Addition of conc. HCl to water
(b) CH4(g) +2O2(g) → CO2(g) + 2H2O(1)
(c) H2O(1) → H2O(g)
(d) CaO(s) + H2O(1) → Ca(OH)2(aq)
Answer
C
Question. A substance which oxidises itself and reduces other is known as –
(a) oxidising agent
(b) reducing agent
(c) both of these
(d) none of these
Answer
B
Question. The oxidation states of P atom in POCl3, H2PO3 and H2P2O6, respectively are
(a) + 5, + 4, + 4
(b) + 5, + 5, + 4
(c) + 4, + 4, + 5
(d) + 3, + 4, + 5
Answer
A
Question. The process of respiration is :
(a) Oxidation reaction which is endothermic
(b) Reduction reaction which is endothermic
(c) Combination reaction which is exothermic
(d) Oxidation reaction which is exothermic
Answer
D
Question. Silver articles become black when exposed to air. It is due to the formation of
(a) Silver oxide
(b) Silver nitrate
(c) Silver chloride
(d) Silve sulphide
Answer
D
Question. A test tube along with calcium carbonate in it initially weighed 30.08 g. A heating experiment was performed on this test tube till calcium carbonate completely decomposed with evolution of a gas. Loss of weight during this experiment was 4.40 g. What is the weight of the empty test tube in this experiment?
(a) 20.08 g
(b) 21.00 g
(c) 24.50 g
(d) 2.008 g
Answer
A
Question. The schematic diagram is given below
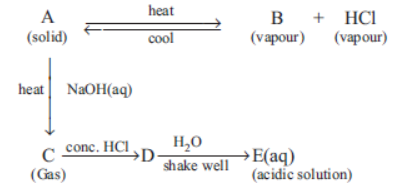
Which of the following is a correct statement ?
(a) A and E are chemically same.
(b) A and D are chemically same.
(c) D and E are chemically same.
(d) C and E are chemically same.
Answer
B
Question. Match chemical reactions given in the List I with the type of chemical reactions given in List II and select the correct answer using the options given below:
(a) A-I, B-V, C-III, D-IV
(b) A-III, B-IV, C-V, D-I
(c) A-IV, B-III, C-V, D-I
(d) A-III, B-I, C-II, D-IV
| List I (Chemical reactions) | List II (Type of Chemical reactions) |
| A. Formation of NH3 from N2 and H2 | I. Decomposition |
| B. Calcination of zinc carbonate. | II. Double displacement |
| C. Reaction of aqueous BaCl2 solution with dilute H2SO4 | III. Combination |
| D. Rancidity of oils | IV. Redox |
| V. Displacement |
Answer
D
Question. A redox reaction is one in which –
(a) both the substances are reduced.
(b) both the substances are oxidised.
(c) an acid is neutralised by the base.
(d) one substance is oxidised while the other is reduced.
Answer
D
Question. When copper turnings are added to silver nitrate solution, a blue coloured solution is formed after some time. It is because, copper –
(a) displaces silver from the solution
(b) forms a blue coloured complex with AgNO3
(c) is oxidised to Cu2+
(d) is reduced to Cu2+
Answer
A
Question. Four students were asked to study the reaction between solution of BaCl2 and sodium sulphate. On mixing the solutions of the two salts in a test tube, they reported their experiments as follows:
(a) The colour of mixture became brown
(b) The solution forms separate layers
(c) A colourless mixture is obtained
(d) A white substance settles at the bottom
Answer
D
Question. 2KClO3 Δ→ 2KCl + 3O2 is a
(a) Decomposition reaction
(b) Combination reaction
(c) Displacement reaction
(d) Redox reaction
Answer
A
Question. Silver turn black due to formation of
(a) Ag2O
(b) Ag2S
(c) Ag2SO4
(d) AgNO3
Answer
B
Question. Which of the following is an antioxidant used in butter?
(a) BSA
(b) BHS
(c) BHT
(d) BHR
Answer
C
Question. Formation of CuO from copper and oxygen denotes
(a) Reduction
(b) Oxidation
(c) Redox reaction
(d) None of these
Answer
B
Question. Rahul took some zinc granules in a test tube and added dil HCl to it. He observed that the colour of Zn granules changed to
(a) Yellow
(b) Brown
(c) Black
(d) White
Answer
D
Question. Copper forms green layer on its surface due to
(a) CuO
(b) CuCO3.Cu(OH)2
(c) CuSO4
(d) CuCl2
Answer
B
QuestionThe rust on iron articles has chemical formula
(a) Fe2O3
(b) FeO
(c) Fe(OH)2
(d) Fe2O3.xH2O
Answer
D
Question. A solution of which of the following compounds is used for whitewashing?
(a) Slaked lime
(b) Quick Lime
(c) Blue vitriol
(d) Limestone
Answer
B
Question. On the basis of evolution or absorption of heat, chemical reactions can be divided into how many types?
(a) Two
(b) Three
(c) Four
(d) One
Answer
A
Question. Which of the following gas is produced when carbon is burnt in air?
(a) Carbon dioxide
(b) Sulphur dioxide
(c) Oxygen
(d) Hydrogen
Answer
A
Question. Electrolysis of water is
(a) Combination reaction
(b) Decomposition reaction
(c) Displacement reaction
(d) None
Answer
B
Question. Lead nitrate Pb(NO3)2 on heating forms solid Lead oxide (PbO) and Nitrogen dioxide gas.
What is the colour of lead oxide and nitrogen dioxide?
(a) White, Colourless
(b) White, Brown
(c) Yellow, Brown
(d) Yellow, Colourless
Answer
C
Question. Silver chloride on exposure to sunlight decomposes into silver and chlorine gas. This property is used in
(a) Heat production as enormous energy is released.
(b) Silver extraction as earth contains silver mainly in silver chloride form.
(c) Photography as grey silver formed produces an image imprint.
(d) None
Answer
C
Question. Quick lime is used in whitewashing because
(a) it is cheap
(b) it forms slaked lime with water which has a nice colour.
(c) it forms slaked lime with water which reacts with atmospheric carbon dioxide to form limestone.
(d) None
Answer
C
Question. Example of a double displacement reaction will be
(a) Metal with a salt
(b) Metal with an acid
(c) Metal with metal
(d) Salt with a salt
Answer
D
Question. Ferrous sulphate solution is green while ferric oxide formed by its decomposition is
(a) red
(b) white
(c) brown
(d) yellow
Answer
C
Question. Corrosion or rusting of iron metal is
(a) Oxidation of iron
(b) Reduction of iron
(c) Displacement of iron
(d) None
Answer
A
Question. To facilitate the electrolysis of water we add a few drops of acids like sulphuric acid because
(a) It acts as a catalyst
(b) It prevents the decomposition of electrodes used.
(c) It makes the water a good conductor of electricity
(d) None
Answer
C
Question. Fried foods are packed with which gas to prevent oxidation of fat?
(a) Oxygen
(b) Nitrogen
(c) Any of the above
(d) None
Answer
B
Question. Which of the following statements about the given reaction are correct?
3Fe (s) + 4H2O (g) → Fe3O4 (s) + 4H2 (g)
(i) Iron metal is getting oxidised
(ii) Water is getting reduced
(iii) Water is acting as reducing agent
(iv) Water is acting as oxidising agent
(a) (i), (ii) and (iii)
(b) (iii) and (iv)
(c) (i), (ii) and (iv)
(d) (ii) and (iv)
Answer
C
Question. Select the oxidising agent for the following reaction:
H2S + I2 → 2HI + S
(a) I2
(b) H2S
(c) HI
(d) S
Answer
A
Question. A substance added to food containing fats and oils is called:
(a) Oxidant
(b) Rancid
(c) Coolant
(d) Antioxidant
Answer
D
Question. The condition produced by aerial oxidation of fats and oils in foods marked by unpleasant smell and taste is called:
(a) antioxidation
(b) reduction
(c) rancidity
(d) corrosion
Answer
C
Question. When SO2 gas is passed through saturated solution of H2S, which of the following reaction occurs?
(a) SO2 + 2H2S → 2H2O + 3S
(b) SO2 + 2H2S → H2O + 3S
(c) SO2 + H2S → H2O + S
(d) SO2 + H2O → SO3 + H2
Answer
A
Question. What are the products formed when iron filings are heated with dilute hydrochloric acid?
(a) Fe (III) chloride and water
(b) Fe (II) chloride and water
(c) Fe (II) chloride and hydrogen gas
(d) Fe (III) chloride and hydrogen gas
Answer
C
Question. A student performs an experiment to form aluminium chloride from aluminium and chlorine.
Which options gives the balanced chemical equation of the reaction? [CBSE Question Bank]
(a) 3Al + 3Cl2 → 3AlCl3
(b) 2Al + Cl2 → 2AlCl
(c) 2Al + 3Cl2 → 2AlCl3
(d) Al + Cl2 → AlCl2
Answer
C
Question. A student adds lead and silver to two different test tubes containing an equal amount of copper sulphate solution. The student observes that the colour of the solution in the test tube with lead changes. What explains the change in the colour of the solution?
(a) A displacement reaction takes place as lead replaces copper from the solution.
(b) Decomposition reaction takes place as copper dissociates from sulphate in the solution.
(c) A double displacement reaction takes place as copper dissociates from sulphate and lead combines with sulphate in the solution.
(d) A combination reaction takes place as lead combines with sulphate in the solution.
Answer
A
Question. The chemical reaction between potassium chloride and silver nitrate is given by the chemical equation. What can be inferred from the chemical equation?
KCl + AgNO3 → AgCl + KNO3
(a) Silver nitrate and potassium undergo a combination reaction to form silver chloride and potassium nitrate.
(b) Silver nitrate and potassium undergo a decomposition reaction to form silver chloride and potassium nitrate.
(c) Silver nitrate and potassium undergo a displacement reaction to form silver chloride and potassium nitrate.
(d) Silver nitrate and potassium chloride undergo double displacement reaction to form silver chloride and potassium nitrate.
Answer
D
Question. A student notices that the bread kept out has a green coloured coating over it after a few days.
What explains the reason for the student’s observation? [CBSE Question Bank]
(a) The oils in the bread reduces and cause the change in the colour of the bread.
(b) Bread comes in contact with atmospheric moisture and corrodes.
(c) The oils in the bread oxidises and causes rancidity.
(d) Comes in contact with the atmospheric nitrogen and a layer deposits over it.
Answer
C
Question. Calcium oxide reacts vigorously with water to produce slaked lime.
CaO (s) + H2O (l) → Ca(OH)2 (aq)
This reaction can be classified as:
(A) Combination reaction
(B) Exothermic reaction
(C) Endothermic reaction
(D) Oxidation reaction
Which of the following is a correct option ?
(a) (A) and (C)
(b) (C) and (D)
(c) (A), (C) and (D)
(d) (A) and (B)
Answer
D
Question. When hydrogen sulphide gas is passed through a blue solution of copper sulphate, a black precipitate or copper sulphide is obtained and the sulphuric acid so formed remains in the solution. The reaction is an example of a :
(a) Combination reaction
(b) Displacement reaction
(c) Decomposition reaction
(d) Double displacement reaction
Answer
D
Question. In a double displacement reaction such as the reaction between sodium sulphate solution and barium chloride solution:
(A) exchange of atoms takes place
(B) exchange of ions takes place
(C) a precipitate is produced
(D) an insoluble salt is produced
The correct option is:
(a) (B) and (D)
(b) (A) and (C)
(c) only (B)
(d) (B), (C) and (D)
Answer
D
In the following question, a statement of Assertion
(A) is followed by a statement of Reason (R). Answer these questions by selecting appropriate option given below:
(a) Both Assertion (A) and Reason (R) are true and Reason (R) is the correct explanation of Assertion (A).
(b) Both Assertion (A) and Reason (R) are true but Reason (R) is not the correct explanation of Assertion (A)
(c) Assertion (A) is true but Reason (R) is false.
(d) Assertion (A) is false but Reason (R) is true.
Question. Assertion (A): Fe2O3 + 2Al → Al2O3 + 2Fe The above chemical equation is an example of displacement reaction.
Reason (R): Aluminium being more reactive than iron, displaces Fe from its oxide.
Answer
A
Question. Assertion (A): Stannous chloride is a powerful oxidising agent which oxidises mercuric chloride to mercury.
Reason (R): Stannous chloride gives grey precipitate with mercuric chloride, but stannic chloride does not do so.
Answer
C
Question. Assertion (A): Calcium Carbonate when heated gives calcium oxide and water.
Reason (R): On heating CaCO3, decomposition reaction takes place.
Answer
D
Question. Assertion (A): Sodium metal is stored under Kerosene.
Reason (R): Metallic sodium melts when exposed to air.
Answer
C
Question. Assertion (A): To dilute sulphuric acid, acid is added to water and not water to acid.
Reason (R): Specific heat of water is quite large.
Answer
A
(a) If both Assertion and Reason are correct and Reason is the correct explanation of Assertion.
(b) If both Assertion and Reason are correct, but Reason is not the correct explanation of Assertion.
(c) If Assertion is correct but Reason is incorrect.
(d) If Assertion is incorrect but Reason is correct.
Question. Assertion : In a reaction Zn(s) + CuSO4 (aq) → ZnSO4 (aq) + Cu(s), Zn is a reductant but itself get oxidized.
Reason : In a redox reaction, oxidant is reduced by accepting electrons and reductant is oxidized by losing electrons.
Answer
A
Question. Assertion : Reaction of sodium sulphate with barium chloride is a precipitation reaction.
Reason : Precipitation reaction produces insoluble salt.
Answer
A
Question. Assertion : Chlorine gas react with potassium iodide solution to form potassium chloride and iodine.
Reason : Chlorine is more reactive than iodine therefore displaces iodine from potassium iodide.
Answer
A
Question. Assertion : Decomposition of vegetable matter into compost is an endothermic reaction.
Reason: Heat is required in an endothermic reaction.
Answer
D
(a) Both ‘A’ and ‘R’ are true and ‘R’ is correct explanation of the assertion.
(b) Both ‘A’ and ‘R’ are true but ‘R’ is not the correct explanation of the assertion.
(c) ‘A’ is true but ‘R’ is false.
(d) ‘A’ is false but ‘R’ is true.
Question. Assertion: The balancing of chemical equation is based on law of conservation of mass.
Reason: Total mass of reactants is equal to total mass of products.
Answer
A
Question. Assertion: The following chemical equation: 2C6H6 + 7/2 O2 → 4CO2 + 3H2O is a balanced chemical equation.
Reason: In a balanced chemical equation, the total number of atoms of each element must be equal on both sides of the equation.
Answer
D
Question. Assertion: Mg + O2 → MgO is skeletal equation.
Reason: The equation is not balanced.
Answer
A
Question. Assertion: Following is a balanced chemical equation for the action of steam on iron:
3Fe + 4H2O → Fe3O4 + 4H2
Reason: The law of conservation of mass holds good for a chemical equation.
Answer
A
Match the Following :
Question. Column II gives type of reaction mention in column I, match them correctly.
| Column I | Column II |
| (A) C + O2 → CO2 | (p) Displacement |
| (B) AgBr hight→ Ag + Br | (q) Combination |
| (C) Zn + CuSO4 → ZnSO4 + Cu | (r) Decomposition |
| (D) CH3CH2OH Cu→ CH3CHO + H2 | (s) Oxidation |
Answer
A → (q) B → (r) C → (p) D → (s)
Question.
| Column I | Column II |
| (A) KClO3 Δ→ | (p) O2 |
| (B) ZnCO3 Δ→ | (q) H2O |
| (C) H2CO3 Δ→ | (r) CO2 |
| (D) C2H6 Δ→ | (s) ZnO |
A B C D
(a) p s, r q, r q, r
(b) p q, r s, r r, p
(c) q, r s, p p,s r
(d) r q s p
Answer
A
Fill in the Blanks :
Question. The digestion of food in the body is an example of ……… reaction.
Answer
Decomposition reaction
Question. Reactions in which energy is absorbed are known as ……….. reactions.
Answer
endothermic
Question. The addition of oxygen to a substance is called ………..
Answer
oxidation
Question. When an element displaces another element from its compound, a ………………… reaction occurs.
Answer
displacement
Question. The new substances produced in a reaction are called as ………………..
Answer
products
True or False :
Question. The number of atoms of each element is conserved in any chemical reaction.
Answer
True
Question. A magnesium ribbon burns with a dazzling flame in air (oxygen) and changes into a white substance, magnesium oxide.
Answer
True
Question. The reaction between nitrogen and hydrogen to give ammonia is an example of a combination reaction.
Answer
True
Question. Rusting is a double decomposition reaction.
Answer
False
Question. The formation of Cu and H2O in the reaction of copper oxide with hydrogen is an example of a redox reaction.
Answer
True
Case Study Based Questions
Read the following and answer any four questions from (i) to (v):
Rahul is a skilled painter. He mixed a white coloured powder, compound X with water. The compound X reacted vigorously with water to produce a compound Y and a large amount of heat. Then, Rahul used the compound Y for white washing the walls. Customer was not satisfied with the work of Rahul as walls were not shining. But Rahul guaranteed him that the walls would shine after 2-3 days. And after 3 days of white wash, the walls became shiny.
Question. Compound X, that Ramesh mixed with water is
(a) Calcium
(b) Calcium oxide
(c) Calcium carbonate
(d) Calcium hydroxide
Answer
B
Question. Name the compound Y, that Ramesh got after mixing X with water.
(a) Calcium
(b) Calcium oxide
(c) Calcium carbonate
(d) Calcium hydroxide
Answer
D
Question. What type of reaction is occurred here?
(a) Decomposition reaction
(b) Displacement reaction
(c) Double displacement reaction
(d) Combination reaction
Answer
D
Question. Which of the following reactions is responsible for shiny finish of the walls?
(a) CaO + H2O → Ca(OH)2
(b) Ca + CO2 → CaCO3
(c) Ca(OH)2 + CO2 → CaCO3 + H2O
(d) CaCO3 + H2O → Ca(OH)2 + CO2
Answer
C
Question. Which of the following is responsible for shiny finish of the walls?
(a) CaCO3
(b) CaO
(c) Ca(OH)2
(d) Ca
Answer
A

We hope you liked the above provided MCQ Questions Chapter 1 Chemical Reactions and Equations Class 10 Science with solutions. If you have any questions please ask us in the comments box below.


