Please refer to the Is Matter Around Us Pure Revision Notes given below. These revision notes have been designed as per the latest NCERT, CBSE and KVS books issued for the current academic year. Students will be able to understand the entire chapter in your class 9th Science book. We have provided chapter wise Notes for Class 9 Science as per the latest examination pattern.
Revision Notes Chapter 2 Is Matter Around Us Pure
Students of Class 9 Science will be able to revise the entire chapter and also learn all important concepts based on the topic wise notes given below. Our best teachers for Grade 9 have prepared these to help you get better marks in upcoming examinations. These revision notes cover all important topics given in this chapter.
Pure Substance & mixture

Elements are made up of one kind of atoms only. Compounds are made up of one kind of molecules only.
Difference between Compound &Mixture
Types of Mixtures
Mixtures can also be grouped
i) on the basis of their physical states:
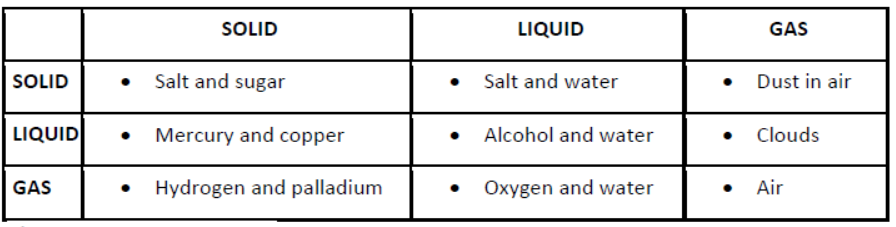
ii) on the basis of miscibility:
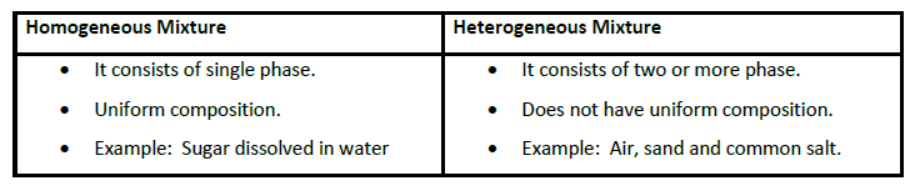
Separating the components of a mixture
The components of a heterogeneous mixture can be separated by
• simple methods like –
hand picking , sieving , & Winnowing
• special techniques like –
i) Evaporation : a mixture of salt and water or sugar and water.
ii) Centrifugation : Butter from curd, Fine mud particles suspended in water.
iii) Decantation (Using separating funnel) : Oil from water.
iv) Sublimation : Camphor from salt,
v) Chromatography : Different pigments from an extract of flower petals.
vi) Distillation and fractional distillation : Separating components of Petroleum
viii) Magnetic separation: Iron pins from sand.
Concentration of Solution
The amount of solute present in a given amount (mass or volume) of solution.
Amount of solute Amount of solute
Concentration of a solution = _________________ OR ____________________
Amount of solvent Amount of solution
The concentration of a solution can be expressed as mass by mass percentage or as mass by volume percentage.
Mass of solute
Mass by mass percentage of a solution = —————— x 100
Mass of solution
Mass of solute
Mass by volume percentage of a solution = —————— x 100
Volume of solution
Types of Solutions
a) on the basis of size of solute particles:
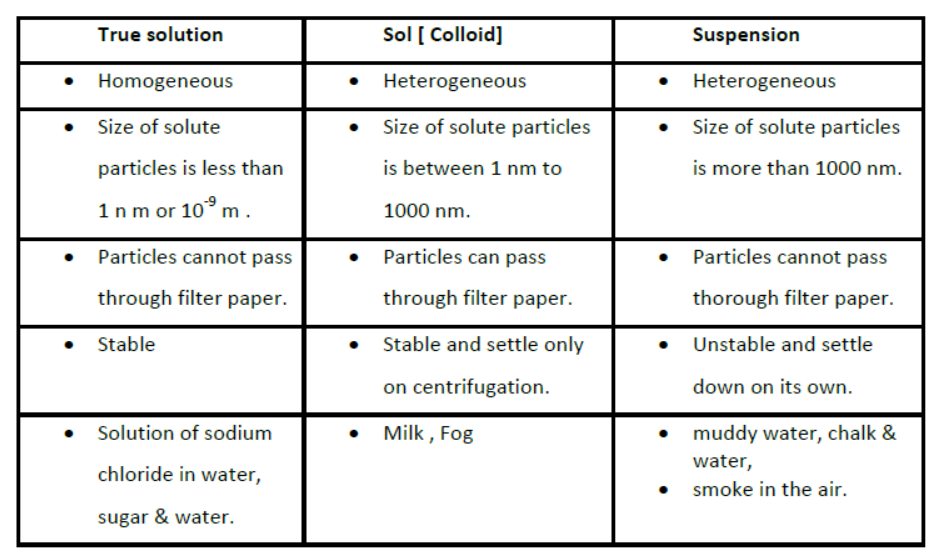
Colloidal solution is a heterogeneous mixture. It consists of two phases:-
(i) Dispersed phase : component present in small proportion
(ii) Dispersion medium : component present in large proportion
The particles of colloid are large enough to scatter a beam of light passing through it and make its path visible. Thus, they show Tyndall effect.
The colloidal particles are moving at random in a zigzag motion in all directions.
This type of zig-zag motion of colloidal particles is called Brownian movement.
b) on the basis of amount of solute:

c) on the basis of nature of solvent

Physical & Chemical Changes
Physical changes: Changes that do not result in the production of a new substance.
• If you melt a block of ice, you still have H2O at the end of the change.
• If you break a bottle, you still have glass.
Examples : melting, freezing, condensing, breaking, crushing, cutting, and bending.
Chemical changes – Changes that result in the production of another substance.
• As in the case of autumn leaves, a change in color is a clue to indicate a chemical change.
• a half eaten apple that turns brown.
Alloys
A material that has metallic properties and is composed of two or more chemical elements of which at least one is a metal .
• These cannot be separated into their components by physical methods.
• However, these are considered as mixture because these show the properties of its constituents and can have variable composition.
The benefit of alloys is that you can combine metals that have varying characteristics to create an end product that is stronger, more flexible, or otherwise desirable to manufacturers.
• Aluminium alloys are extensively used in the production of automotive engine parts.
• Copper alloys have excellent electrical and thermal performance, good corrosion resistance, high ductility and relatively low cost.
• Stainless steel alloys are used for many commercial applications such as watch straps, cutlery etc.
• Titanium alloys have high strength, toughness and stiffness & are used in aerospace structures .



