Please refer to Alcohols Phenols and Ethers HOTs Class 12 Chemistry provided below with solutions. All HOTs for Class 12 Chemistry with answers provided below have been designed as per the latest syllabus and examination petter issued by CBSE, NCERT, KVS. Students of Standard 12 Chemistry should learn the solved HOTS for Class 12 Chemistry provided below to gain better marks in examinations.
Alcohols Phenols and Ethers Class 12 Chemistry HOTs
Question. Sodium salt of benzene sulphonic acid on fusion with caustic soda gives
(a) Benzene
(b) Phenol
(c) Thiophenol
(d) Benzoic acid
Answer
B
Question. Which of the following is dihydric alcohol ?
(a) Glycerol
(b) Ethylene glycol
(c) Catechol
(d) Resorcinol
Answer
B
Question. Acid catalyzed hydration of alkenes except ethene leads to the formation of
(a) primary alcohol
(b) secondary or tertiary alcohol
(c) mixture of primary and secondary alcohols
(d) mixture of secondary and tertiary alcohols
Answer
B
Question. Butane-2-ol is
(a) primary alcohol
(b) secondary alcohol
(c) tertiary alcohol
(d) aldehyde
Answer
B
Question. Cresol has
(a) Alcoholic – OH
(b) Phenolic – OH
(c) – COOH
(d) – CHO
Answer
B
Question. By which of the following methods alcohol can be prepared in excellent yield?
(a) From alkenes
(b) By hydroboration-oxidation
(c) From carbonyl compounds
(d) From Grignard reagent
Answer
B
Question. How many isomers of C5H11OH will be primary alcohols ?
(a) 5
(b) 4
(c) 2
(d) 3
Answer
B
Question. The IUPAC name of
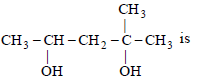
(a) 1, 1-dimethyl-1, 3-butanediol
(b) 2-methyl-2, 4-pentanediol
(c) 4-methyl-2, 4-pentanediol
(d) 1, 3, 3-trimethyl-1, 3-propanediol
Answer
B
Question. Number of metamers represented by molecular formula C4H10O is
(a) 4
(b) 3
(c) 2
(d) 1
Answer
B
Question. The structural formula of cyclohexanol is
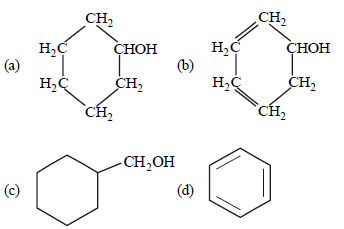
Answer
A
Question. How many alcohol(s) with molecular formula C4H10O are chiral in nature?
(a) 1
(b) 2
(c) 3
(d) 4
Answer
A
Question. IUPAC name of m-cresol is ___________
(a) 2-methylphenol
(b) 3-chlorophenol
(c) 3-methoxyphenol
(d) benzene-1, 3-diol
Answer
A
Question. Which of the following will not form phenol or phenoxide ?
(a) C6H5N2Cl
(b) C6H5SO3Na
(c) C6H5Cl
(d) C6H5CO2H
Answer
D
Question. IUPAC name of the compound

(a) 1-methoxy-1-methylethane
(b) 2-methoxy-2-methylethane
(c) 2-methoxypropane
(d) isopropylmethyl ether
Answer
C
Question. Which of the following compounds is aromatic alcohol?

(a) A, B, C, D
(b) A, D
(c) B, C
(d) A
Answer
C
Question. In which of the following structures hydroxyl group is attached to sp2 carbon atom?

Answer
C
Question. Alkenes convert into alcohols by
(a) hydrolysis by dil. H2SO4
(b) hydration of alkene by alkaline KMnO4
(c) hydrolysis by water vapours and conc. HNO3
(d) hydration of alkene by aqueous KOH
Answer
B
Question. Benzyl alcohol is obtained from benzaldehyde by
(a) Fittig’s reaction
(b) Cannizzaro’s reaction
(c) Kolbe’s reaction
(d) Wurtz’s reaction
Answer
B
Question. Give IUPAC name of the compound given below

(a) 2-Chloro-5-hydroxyhexane
(b) 2-Hydroxy-5-chlorohexane
(c) 5-Chlorohexane-2-ol
(d) 2-Chlorohexan-5-ol
Answer
C
Question. An example of a compound with functional group – O – is :
(a) acetic acid
(b) methyl alcohol
(c) diethyl ether
(d) acetone
Answer
C
Question. In the reduction
R — CHO + H2 → RCH2OH the catalyst used is :
(a) Ni
(b) Pd
(c) Pt
(d) Any of these
Answer
D
Question. The compound HOCH2 – CH2OH is
(a) ethane glycol
(b) ethylene glycol
(c) ethylidene alcohol
(d) dimethyl alcohol
Answer
B
Question. Ethylene reacts with Baeyer’s reagent to give
(a) ethane
(b) ethyl alcohol
(c) ethylene glycol
(d) None of these
Answer
C
Question. Which of the following reacts with NaOH to give an alcohol?
(a) Propene
(b) Butene
(c) Ethanal
(d) Methanal
Answer
D
Question. Ethyl alcohol is industrially prepared from ethylene by
(a) Permanganate oxidation
(b) Catalytic reduction
(c) Absorbing in H2SO4 followed by hydrolysis
(d) All the three
Answer
C
Question. The characteristic grouping of secondary alcohols is

Answer
B
Question. Ethyl alcohol can be prepared from Grignard reagent by the reaction of :
(a) HCHO
(b) R2CO
(c) RCN
(d) RCOCl
Answer
A
Question. Which of the following is an example of unsymmetrical ether?
(a) C2H5OC2H5
(b) C6H5OC6H5
(c) C6H5OC2H5
(d) CH3OCH3
Answer
C
Question. Isopropyl alcohol is obtained by reacting which of the following alkenes with concentrated H2SO4 followed by boiling with H2O?
(a) Ethylene
(b) Propylene
(c) 2-Methylpropene
(d) Isoprene
Answer
B
Question. Which of the following are benzylic alcohols?
(i) C6H5 – CH2 – CH2OH
(ii) C6H5 – CH2OH

(a) (i) and (ii)
(b) (ii) and (iii)
(c) (i), (ii) and (iv)
(d) (i) and (iv)
Answer
B
Question. Which of the following compounds is resistant to nucleophilic attack by hydroxyl ions?
(a) Methyl acetate
(b) Acetonitrile
(c) Acetamide
(d) Diethyl ether
Answer
D
Question. The major organic product in the reaction,
CH3 — O — CH(CH3)2 + HI → Product is

Answer
C
Question. Formation of diethyl ether from ethanol is based on a
(a) dehydration reaction
(b) dehydrogenation reaction
(c) hydrogenation reaction
(d) heterolytic fission reaction
Answer
A
Question. An aromatic ether is not cleaved by HI even at 525 K. The compound is
(a) C6H5OCH3
(b) C6H5OC6H5
(c) C6H5OC3H7
(d) Tetrahydrofuran
Answer
B
Question. Methylphenyl ether can be obtained by reacting
(a) phenolate ions and methyl iodide
(b) methoxide ions and bromobenzene
(c) methanol and phenol
(d) bromo benzene and methyl bromide
Answer
A
Question. Diethyl ether can be decomposed by heating with
(a) HI
(b) NaOH
(c) Water
(d) KMnO4
Answer
A
Question. The cleavage of an aryl-alkyl ether with cold HI gives :
(a) alkyl iodide and water
(b) aryl iodide and water
(c) alkyl iodide, aryl iodide and water
(d) phenol and alkyl iodide
Answer
D
Question.. When 2-methoxypropane is heated with HI, in the mole ratio 1 : 1, the major products formed are
(a) methanol and 2-iodopropane
(b) methyl iodide and 2-propanol
(c) methyl iodide and 2-iodopropane
(d) methanol and 2-propanol
Answer
B
STATEMENT TYPE QUESTIONS
Question. Read the following statements and choose the correct option.
(i) Ethanol on dehydration at 443 K gives ethene
(ii) Ethanol on dehydration at 413 K gives diethyl ether
(iii) Only primary alcohols on dehydration give ethers.
(iv) Secondary and tertiary alcohols on dehydration give ethers having 2° and 3° carbon attached with O atom.
(a) TTFF
(b) TFTF
(c) TTTF
(d) FTTF
Answer
C
Question. Which of the following are correct statement(s) ?
(i) Polar nature of O–H bond is responsible for acidic character of alcohols.
(ii) Acidic strength of alcohols follow the order 1° > 2° > 3°.
(iii) Alcohols are stronger acids than water.
(iv) Alcohols also react as Bronsted base.
(a) (i), (ii) and (iii)
(b) (i), (ii) and (iv)
(c) (ii), (iii) and (iv)
(d) (i), (iii) and (iv)
Answer
B
Question. Which of the following statements are correct ?
(i) Ethanol mixed with methanol is called denatured alcohol.
(ii) Excess of methanol in body may cause blindness.
(iii) In the body methanol is oxidised to methanoic acid.
(iv) A methanol poisoned patient is treated by giving intravenous injections of ethanoic acid.
(a) (i), (ii) and (iii)
(b) (ii), (iii) and (iv)
(c) (i) and (v)
(d) (i), (iii) and (iv)
Answer
A
Question. When an alcohol is prepared by reaction of ethylmagnesiumbromide with 2–pentanone, product formed does not rotate plane polarised light. For this reaction which of the following statement(s) is/are correct ?
(i). Product formed is achiral.
(ii) Racemic mixture is formed.
(a) Both statements (i) and (ii) are correct.
(b) Statement (i) is correct only.
(c) Statement (ii) is correct only.
(d) Both statements (i) and (ii) are incorrect.
Answer
C
Question. Which of the following statements are correct ?
(i) In phenols, the —OH group is attached to sp2 hybridised carbon of an aromatic ring
(ii) The carbon – oxygen bond length (136 pm) in phenol is slightly more than that in methanol
(iii) Partial double bond character is due to the conjugation of unshared electron pair of oxygen with the aromatic ring.
(iv) sp2 hybridised state of carbon to which oxygen is attached.
(a) (i), (ii) and (v)
(b) (i), (ii) and (iii)
(c) (i), (iii) and (iv)
(d) (i) and (iv)
Answer
C
Question. Which of the following statements are correct ?
(i) Alcohols react as nucleophiles in the reactions involving cleavage of O–H bond.
(ii) Alcohols react as electrophiles in the reactions involving cleavage of O–H bond.
(iii) Alcohols react as nucleophile in the reaction involving cleavage of C–O bond.
(iv) Alcohols react as electrophiles in the reactions involving C–O bond.
(a) (i) only
(b) (i) and (iv)
(c) (ii) and (iii)
(d) (ii) only
Answer
B